Sensores de oximetría de pulso
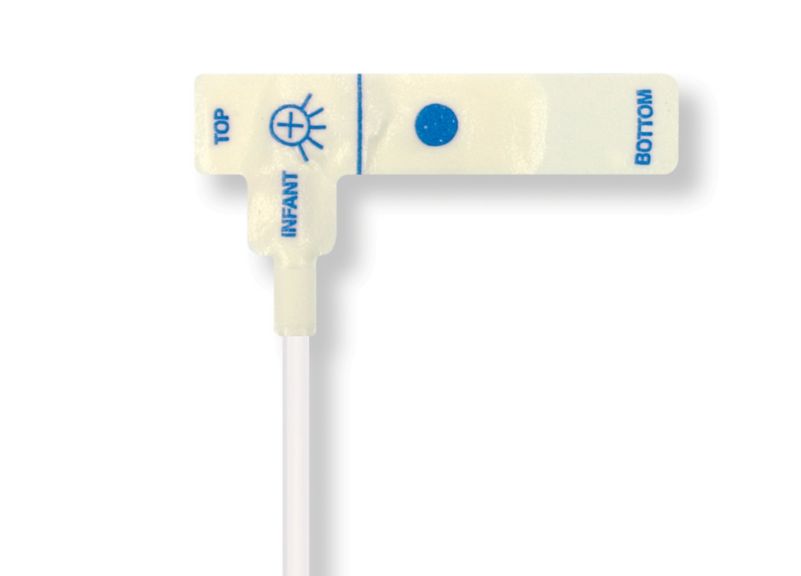
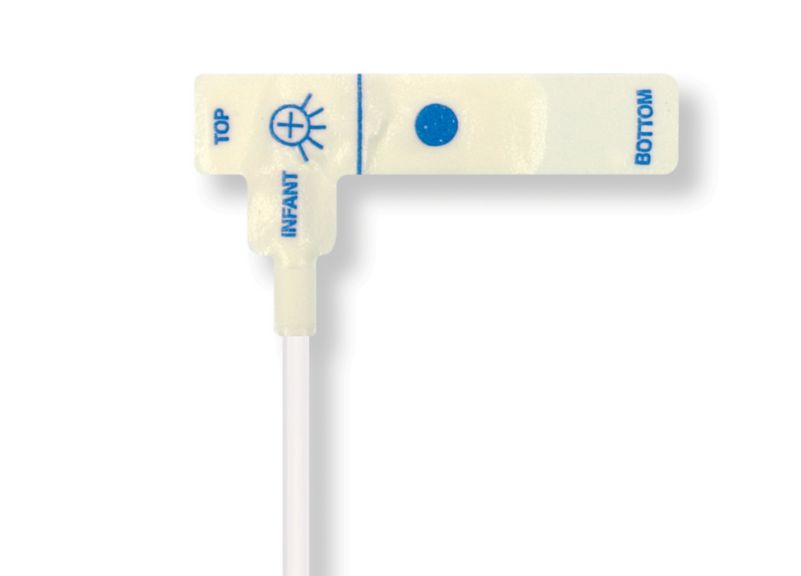
Sensores de pulsioximetría desechables para niños
Sensores de pulsioximetría desechables para niños. Cómodos al reposicionar (cinta suave de calidad médica). Menos trauma cutáneo derivado del adhesivo si es necesario: recién nacidos, pacientes geriátricos o con quemaduras, adaptados al entorno
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
Lista de referencias cruzadas de EnviteC | Soporte técnico
Sensores de SpO2 desechables para niños. Estos sensores no conllevan el riesgo de contaminación cruzada causada por productos médicos que se reutilizan de un paciente a otro.
La alta flexibilidad, adaptabilidad y biocompatibilidad del material blando aplicado hace que este tipo de sensor sea ideal para aplicaciones donde el paciente necesita una monitorización prolongada a largo plazo.
EnviteC by Honeywell
Certificate
Others
Instructions for Use
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión