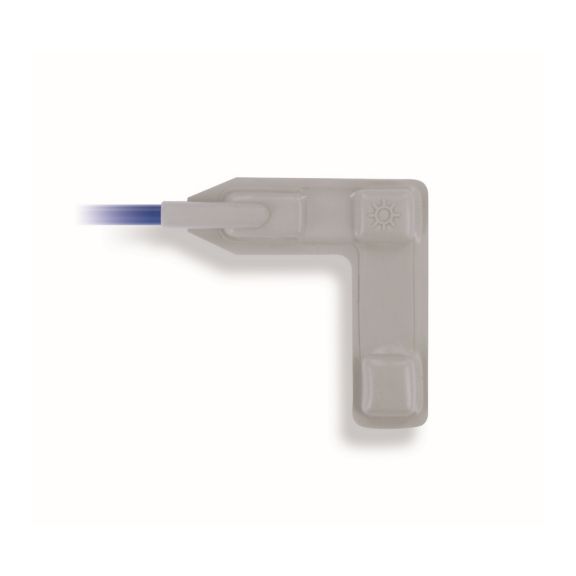
Pulsoximetrie-Sensoren
Wrap-Sensoren
Wiederverwendbare Wrap-Pulsoximetriesensoren für Neugeborene bis Erwachsene. Flexible Anwendung. Leicht zu reinigen
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC-Querverweisliste | Technische Unterstützung
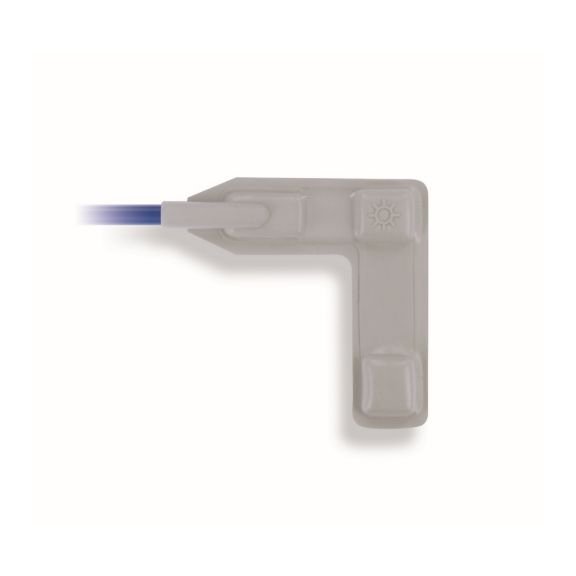
Wiederverwendbare Wrap-Pulsoximetriesensoren zum Wickeln. Leicht zu reinigende und flexible Anwendungen
EnviteC by Honeywell
Certificate
Others
Bitte melden sie sich an, um die für den kauf verfügbaren teilenummern basierend auf ihrem konto anzuzeigen Anmelden
Bitte melden sie sich an, um die für den kauf verfügbaren teilenummern basierend auf ihrem konto anzuzeigen Anmelden