Pulsoximetrie-Sensoren
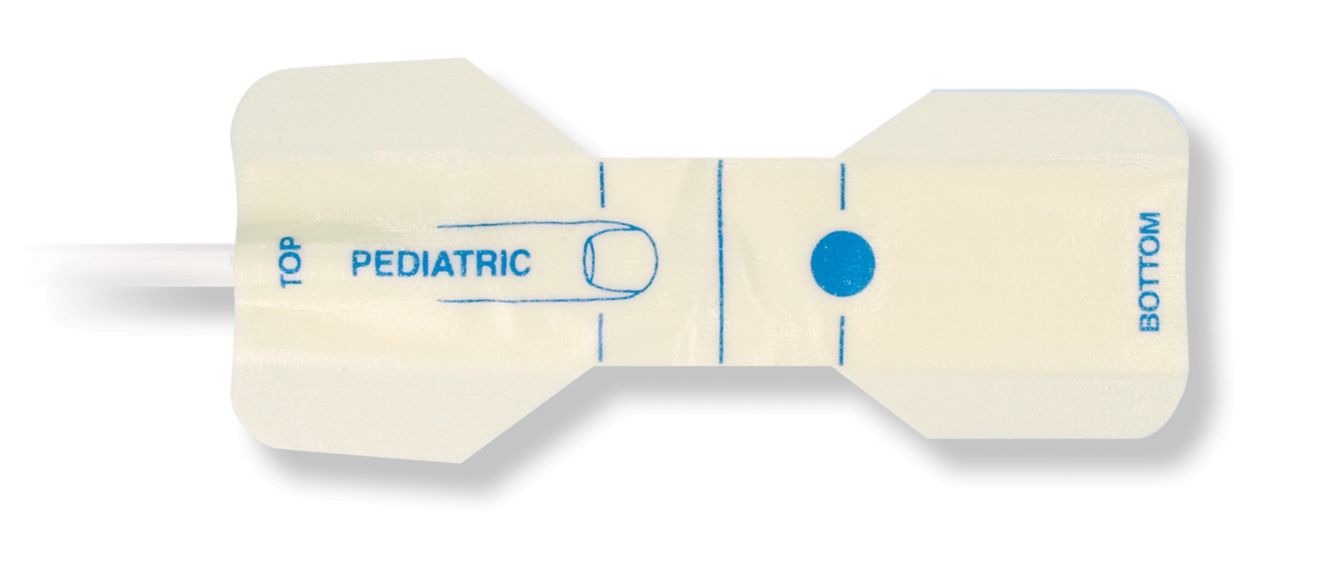
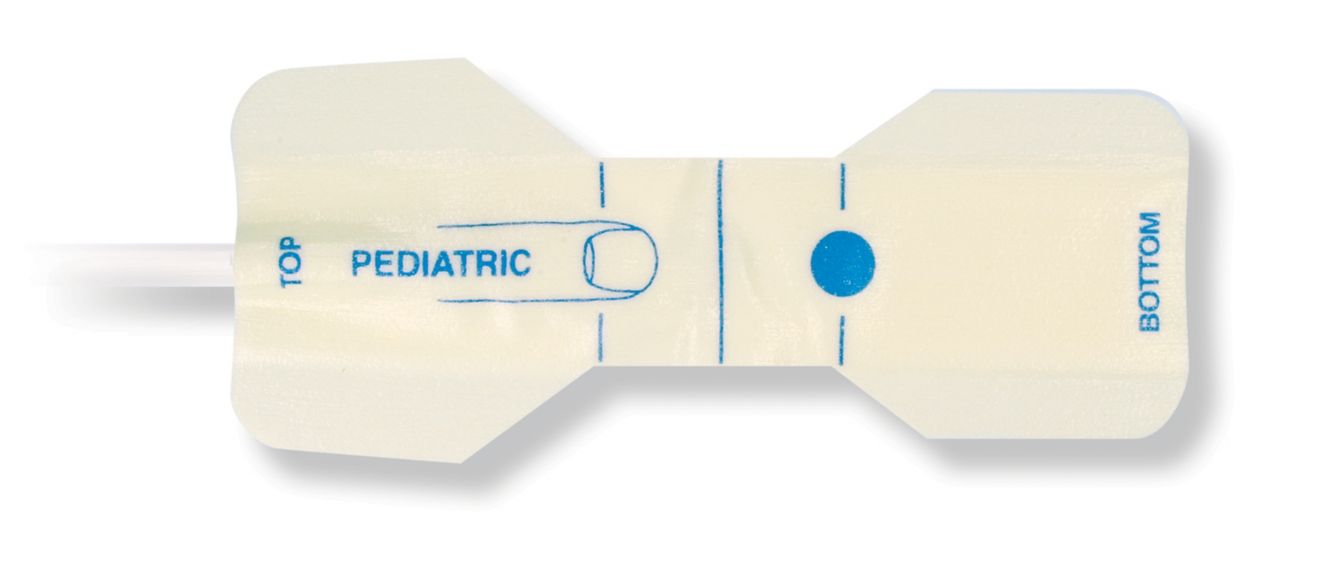
Pediatric Einweg-Pulsoximetriesensoren
Einweg-Pulsoximetriesensoren für Kinder. Komfort bei Neupositionierung (weiches medizin. Klebeband). Weniger Hauttrauma, sofern erforderlich – Neugeborene, geriatrische Patienten, Patienten mit Brandverletzungen und angepasst an die Umgebung
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC-Querverweisliste | Technische Unterstützung
Einweg-SpO2-Sensoren für Kinder. Diese Sensoren bergen nicht das Risiko einer Kreuzkontamination durch Medizinprodukte, die von Patient zu Patient wiederverwendet werden.
Die hohe Flexibilität, Anpassungsfähigkeit und Biokompatibilität des verwendeten weichen Materials macht diesen Sensortyp ideal für Anwendungen, bei denen der Patient über einen längeren Zeitraum überwacht werden muss.
EnviteC by Honeywell
Certificate
Others
Instructions for Use
Bitte melden sie sich an, um die für den kauf verfügbaren teilenummern basierend auf ihrem konto anzuzeigen Anmelden
Bitte melden sie sich an, um die für den kauf verfügbaren teilenummern basierend auf ihrem konto anzuzeigen Anmelden