pulse oximetry sensors
Wrap
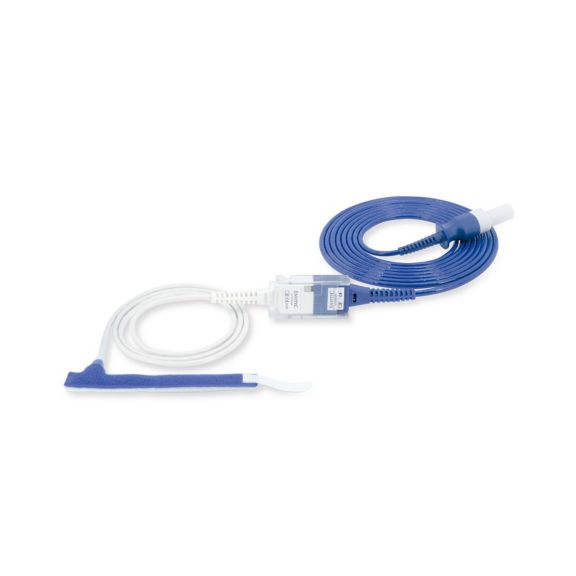
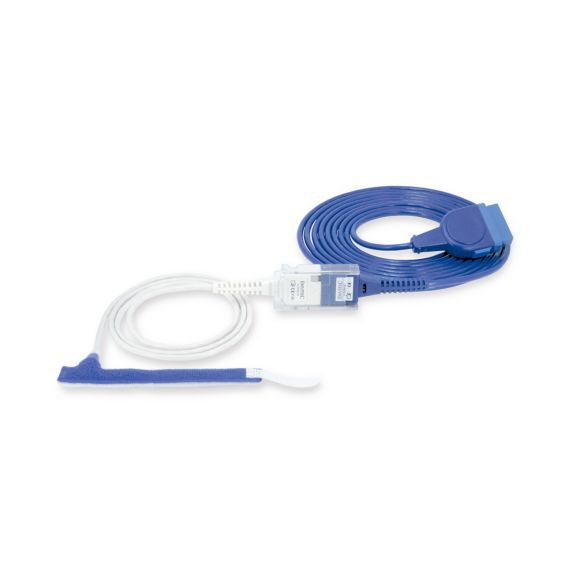
Reuseable wrap pulse oximetry sensors for neonates to adults. Flexible application. Easy to clean.
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Resusable wrap pulse oximetry sensors. Easy to clean and flexible applications.
EnviteC by Honeywell
Others
- Industry
- Healthcare
Others
- Industry
- Healthcare
- Industry : Healthcare
Please sign in to access more documents.
Once signed in, you may be able to access additional documents for your account.
Certificate
Others
Instructions for Use
Please sign in to view part numbers available for purchase based on your account Sign In