Pulsoximetrie-Sensoren
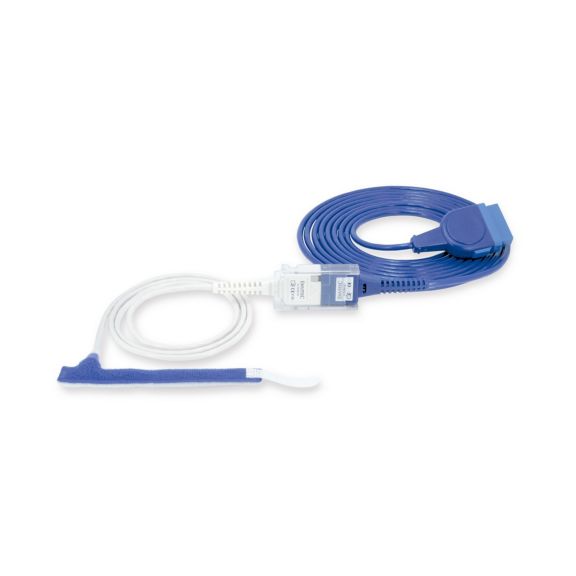
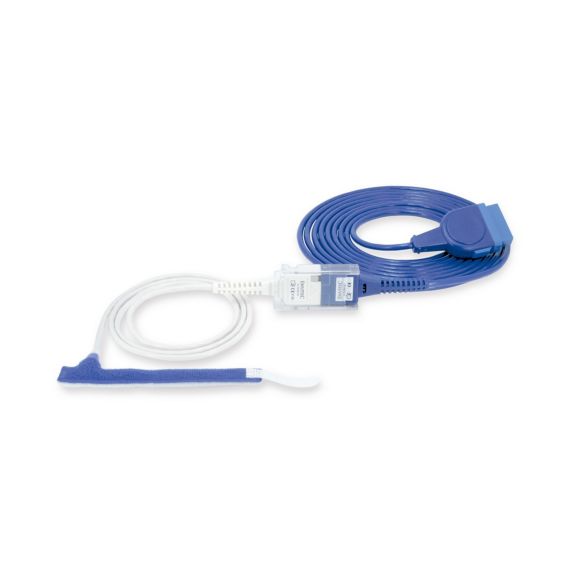
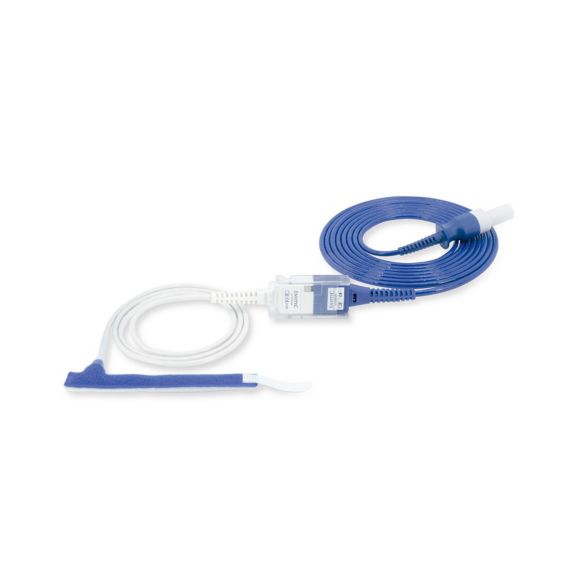
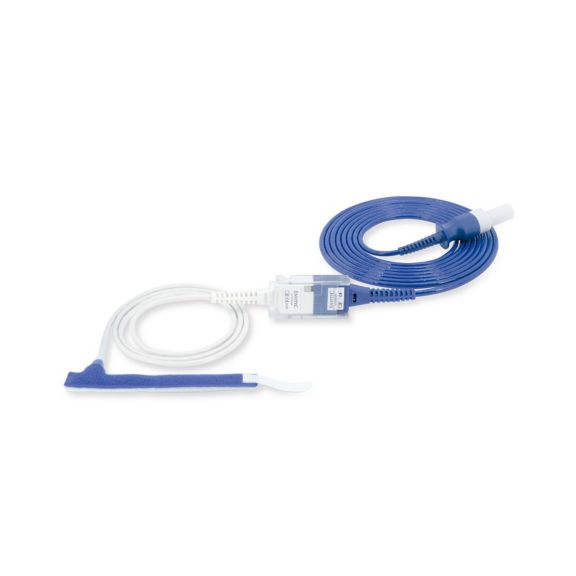
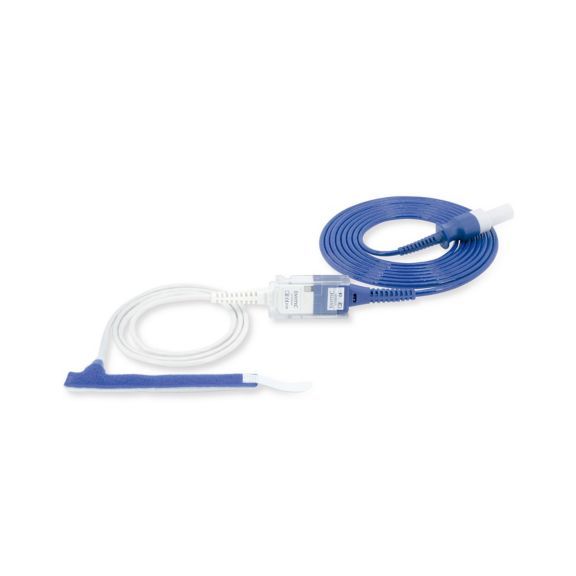
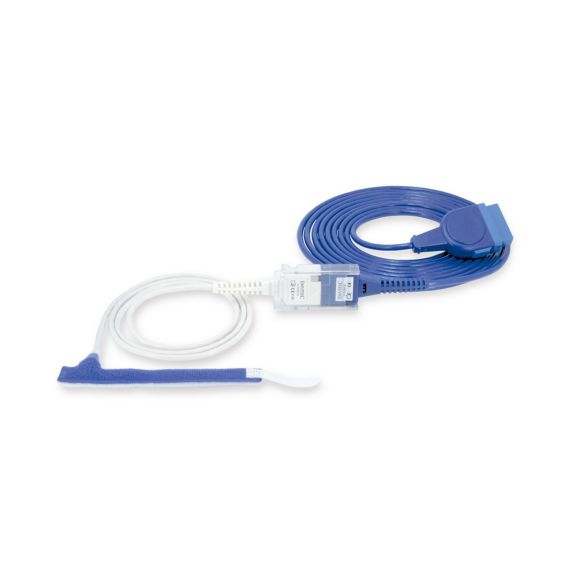
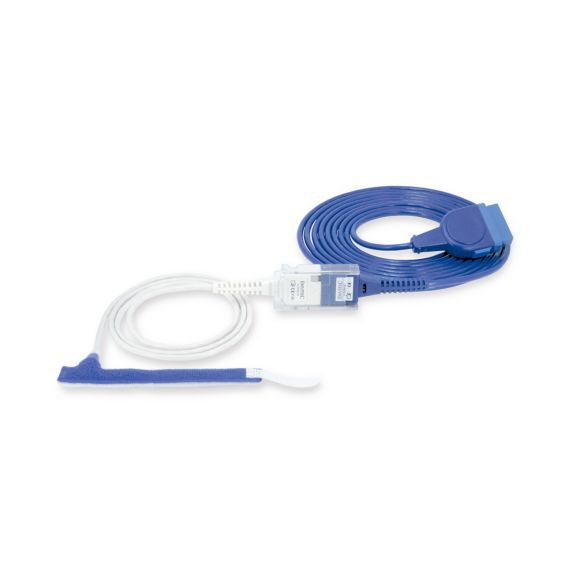
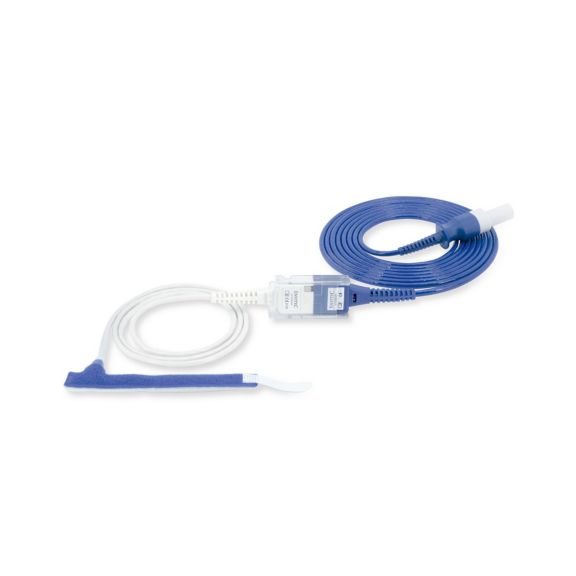
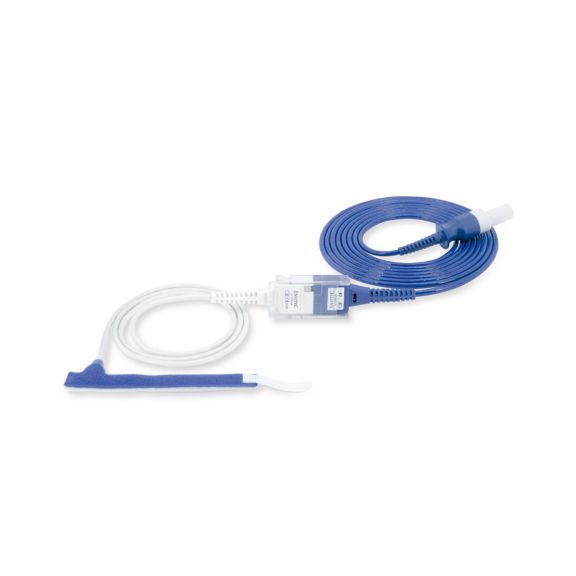
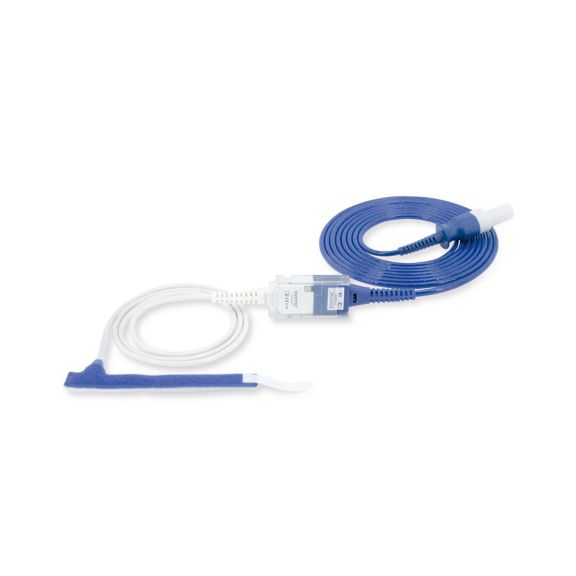
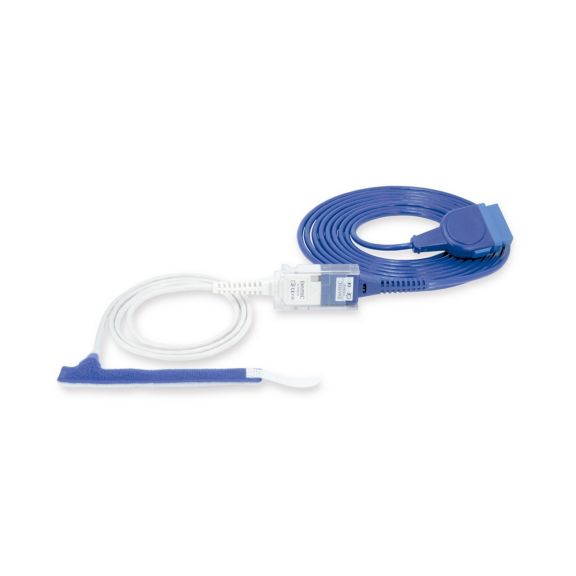
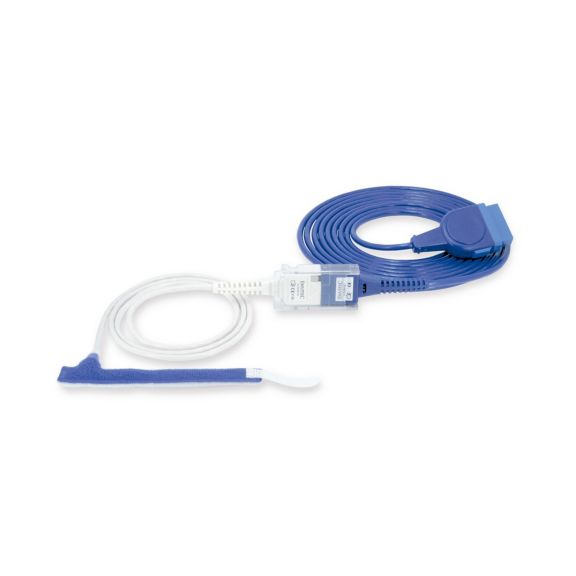
Wrap-Sensor
Wiederverwendbare Wrap-Pulsoximetriesensoren für Neugeborene bis Erwachsene. Flexible Anwendung. Leicht zu reinigen
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.

EnviteC-Querverweisliste | Technische Unterstützung
Wiederverwendbare Wrap-Pulsoximetriesensoren. Leicht zu reinigende und flexible Anwendungen.
EnviteC by Honeywell
Zertifikat
Andere
Instructions for Use
Bitte melden sie sich an, um die für den kauf verfügbaren teilenummern basierend auf ihrem konto anzuzeigen Anmelden
Bitte melden sie sich an, um die für den kauf verfügbaren teilenummern basierend auf ihrem konto anzuzeigen Anmelden