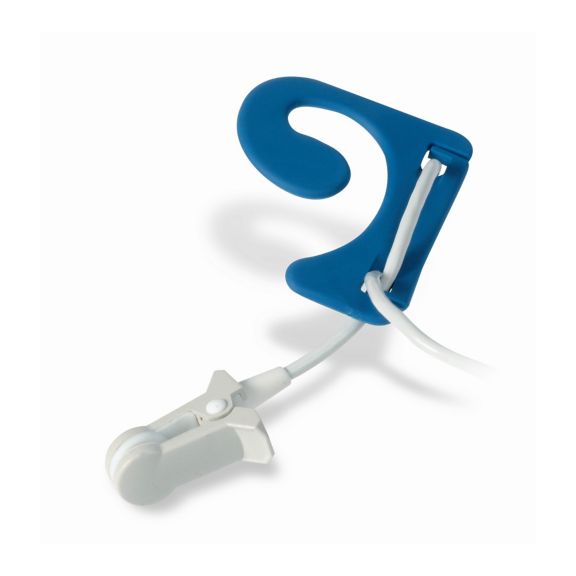
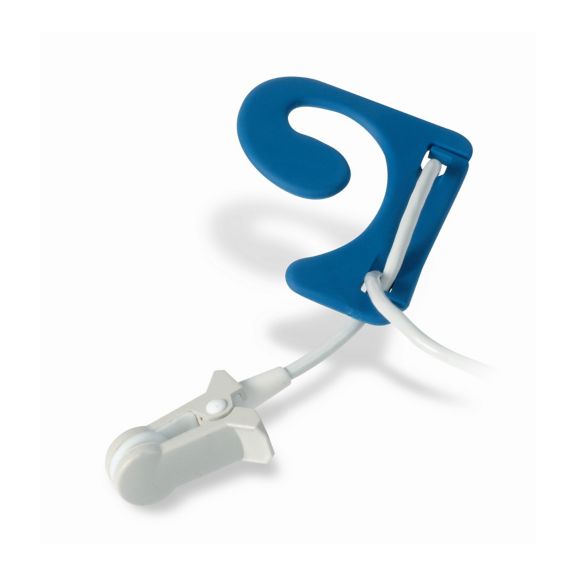
Pulsoximetrie-Sensoren
Ohrsensoren
Die wiederverwendbaren Ear-Clip-Pulsoximetriesensoren bieten Patientenkomfort, leicht und frei beweglich. Workflow-Optimierung – einfache Applkation und bleibt an Ort und Stelle. Hohe Zuverlässigkeit – schnelle Reaktion und weniger Bewegungsartefakte
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC-Querverweisliste | Technische Unterstützung
Die Verwendung einer SpO2-Sonde zur nicht-invasiven Überwachung der arteriellen Sauerstoffsättigung gehört in den meisten Krankenhäusern und anderen medizinischen Umgebungen zum Standard der Versorgung.
Die am häufigsten verwendeten Sonden werden an der Fingerspitze des Patienten angebracht. Es gibt jedoch auch andere Möglichkeiten der Sondenpositionierung, die eine gute klinische Praxis darstellen und Alternativen bieten, insbesondere wenn die Bedingungen schlecht sind.
Der Ear-Clip-Sensor ist ein bewährter und klinisch genutzter Messpunkt. In Bezug auf die Messgenauigkeit ist der Ear-Clip mit dem Finger-Clip vergleichbar – in besonderen Fällen sogar überlegen.
Bei Kindern und Erwachsenen kann das Ohrläppchen der bevorzugte Messort sein, da Störartefakte durch Bewegungen deutlich geringer sind.
EnviteC by Honeywell
Brochure
Certificate
Others
Bitte Melden Sie sich an, um die zum Kauf verfügbaren Teilenummern basierend auf Ihrem Konto anzuzeigen melden Sie sich an,
Bitte Melden Sie sich an, um die zum Kauf verfügbaren Teilenummern basierend auf Ihrem Konto anzuzeigen melden Sie sich an,