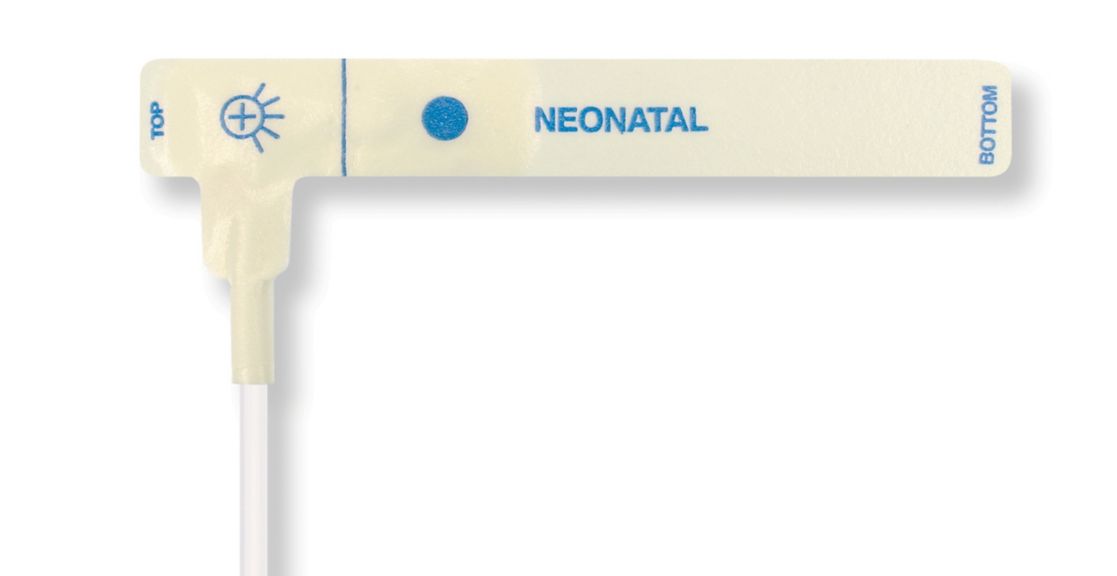
맥박 산소 측정 센서
신생아용 일회용 맥박 산소측정 센서
신생아용 일회용 맥박 산소측정 센서. 위치 조정의 편안함(소프트 의료용 테이프). 필요한 경우 피부 유착성 외상 감소 – 신생아, 노인 환자, 화상을 입은 환자 및 환경에 적응한 환자
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
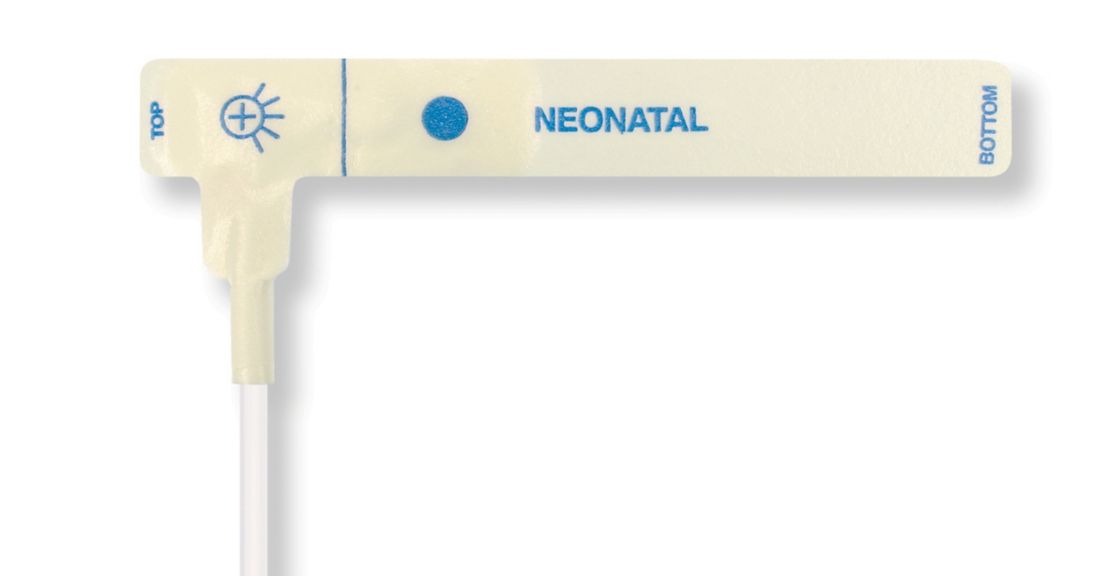
신생아용 일회용 SpO2 센서. 이러한 센서는 환자에게 재사용되는 의료 제품으로 인한 교차 오염의 위험을 수반하지 않습니다.
도포된 부드러운 재질의 높은 유연성, 적응성 및 생체 적합성으로 인해 이 센서 유형은 환자가 장기간 모니터링을 필요로 하는 분야에 이상적입니다.
Honeywell의 EnviteC