pulse oximetry sensors
包裹式传感器
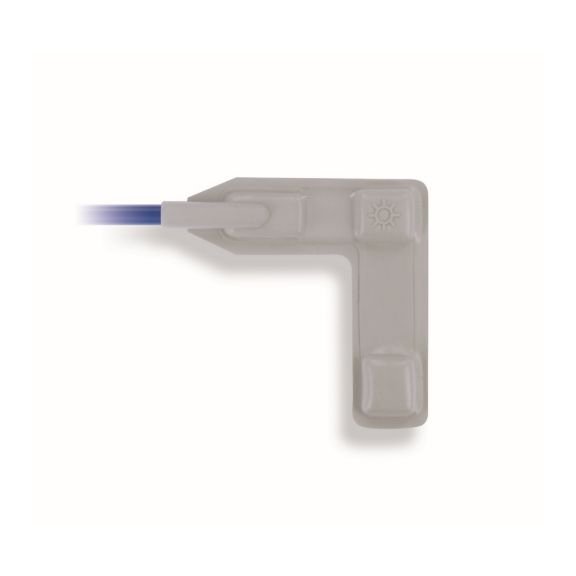
可重复使用的包裹式脉搏血氧测定传感器,适合从新生儿到成人的各类人群。灵活应用。易于清洗
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
请登录以访问更多文档。
登录后,您可以访问账户中的其他文档。
Africa
Asia Pacific
Europe
Latin America
Middle East
North America
您可以为此帐户设置首选货币
选择货币
更改货币将导致您当前的购物车被删除。单击“确定”继续
要保留当前的购物车,请单击“关闭”,然后在更改货币之前保存您的购物车
切换帐户将更新您可用的产品目录。切换帐户时,您当前的购物车不会移动到您选择的新帐户。如果您再次登录此帐户,您当前的购物车将可用。
| 帐户# | 帐户名称 | 城市 | 邮政编码 |
|---|
You are browsing the product catalog for
You are viewing the overview and resources for
pulse oximetry sensors
Once signed in, you may be able to access additional documents for your account.



登录后,您可以访问账户中的其他文档。
请 登录后即可查看根据您的帐户可购买的零件编号 登入
| IMG | IMG | IMG | IMG | IMG | |
|---|---|---|---|---|---|
| Product NameSKU | Product NameSKU | Product NameSKU | Product NameSKU | Product NameSKU | |
| Description | X | X | X | X | X |
| Description | X | X | X | X | X |
| Description | X | X | X | X | X |
| Description | X | X | X | X | X |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
| X | BRV900~51400955-100 |
订阅我们!
立即注册以通过电话、邮件等电子通信方式接收霍尼韦尔产品更新、技术信息、新品发布、活动和新闻、问卷调查、特别优惠等相关主题的独家营销信息。
Copyright © 2026 Honeywell International Inc
