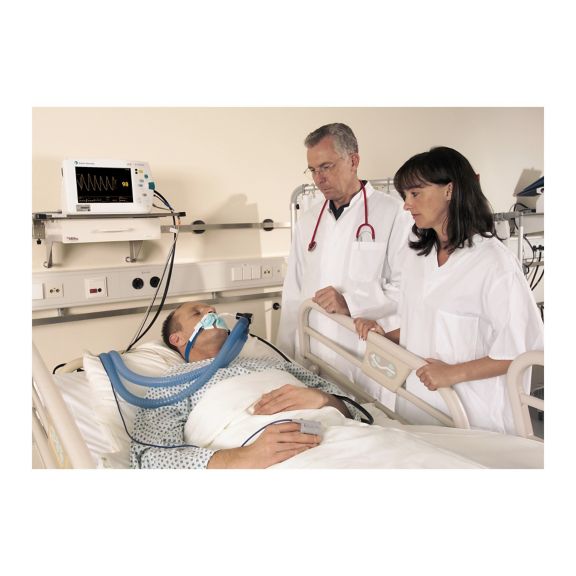
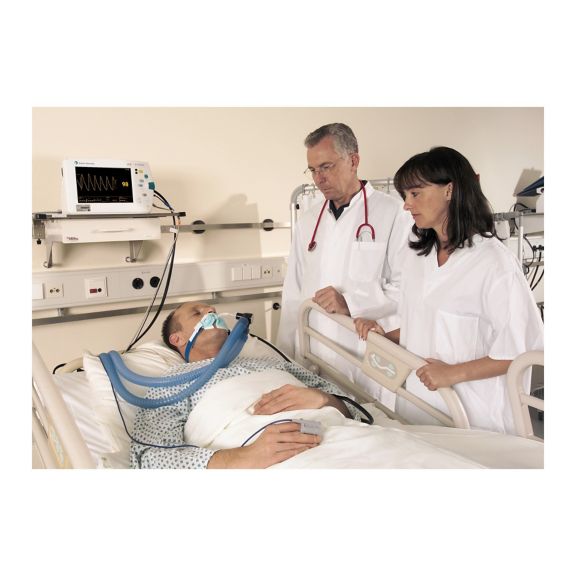
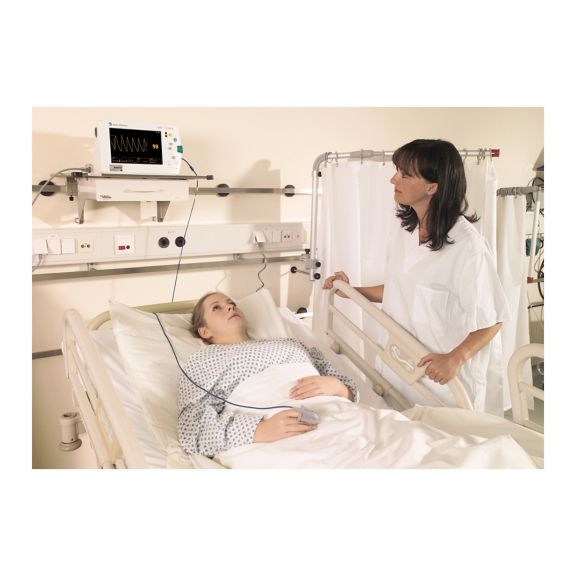
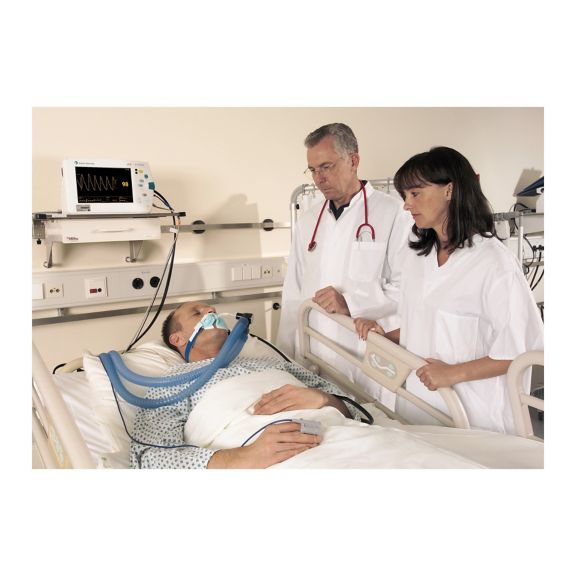
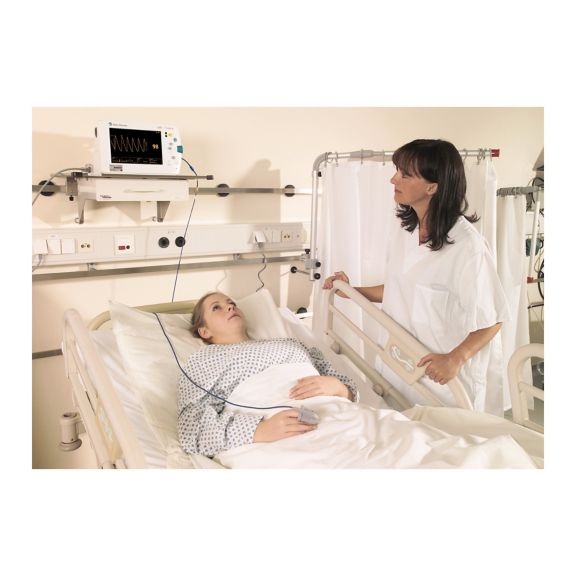
脉搏血氧传感器
SoftTip®
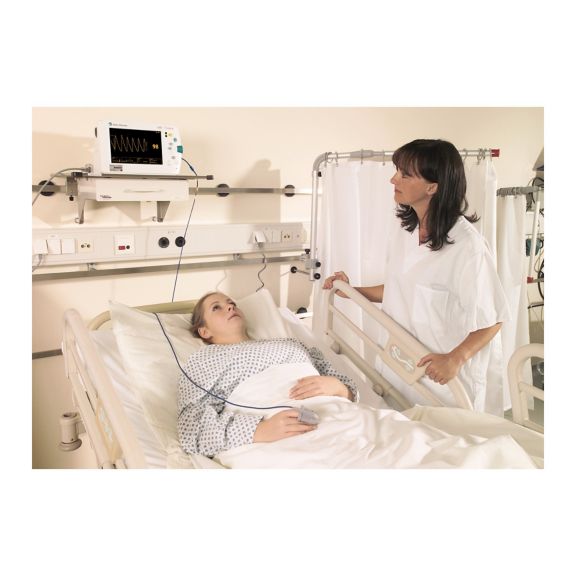
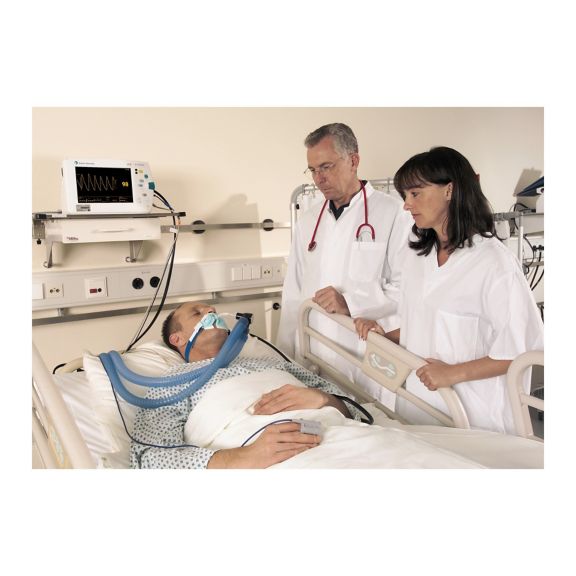
SoftTip® 可重复使用的脉搏血氧测定传感器,操作简单,方便清洁并能彻底消毒。最大程度耐受机械应力。可针对不同应用提供不同尺寸,耐磨且使用舒适
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
一个长期可重复使用的手指传感器,可完全浸没在清洁液体中,方便清洁。同时,可承受最大的机械应力。
独特的产品设计可确保极高的患者舒适度和超精确的测量结果。保证与多数知名厂商的常用监控设备兼容。
霍尼韦尔的 EnviteC