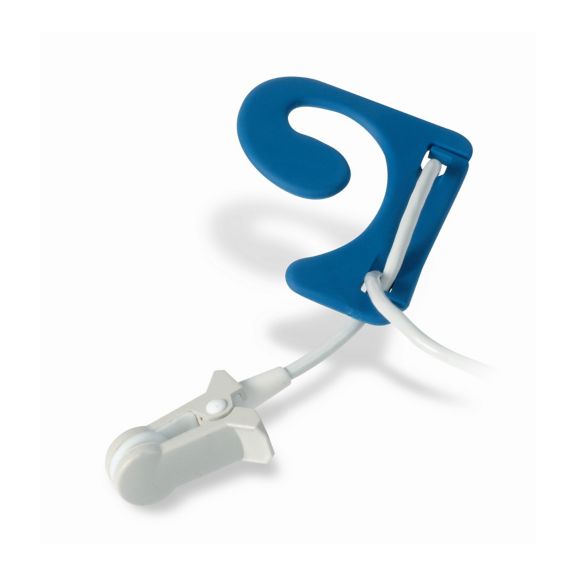
pulse oximetry sensors
耳传感器
耳夹式可重复使用的脉搏血氧测定传感器,提高患者使用舒适度——轻盈、自由移动。优化工作流程——易用且能保持原位。高可靠性——快速响应,减少运动伪影
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
在大多数医院设置和其他医疗环境中,使用 SpO2 探头对动脉血氧饱和度进行无创监测是标准护理。
最常用的探头放在患者的指尖。但是,仍可通过其他探针定位方法提供良好的改善临床实践,并提供替代方法,尤其是在情况不佳时。
耳夹式传感器是一个经过验证和临床使用的测量点。特别是临床准确性方面,耳夹不仅与指夹相当,在某些情况下甚至优先被报告。
对于儿童和成人,考虑到减少运动伪影的干扰,耳垂可能是首选测量点。
霍尼韦尔的 EnviteC
请登录以访问更多文档。
登录后,您可以访问账户中的其他文档。