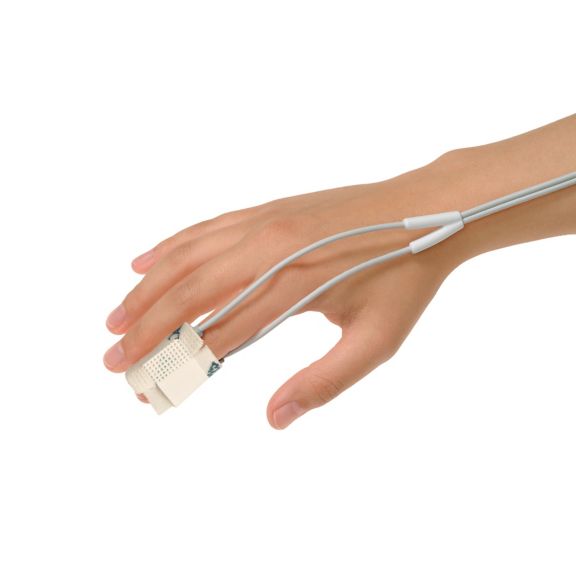
pulse oximetry sensors
Y Sensors
Y reusable pulse oximetry sensors for multi-site applications. Design for neonates to adults. Flexible application. Easy to clean.
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Y reusable pulse oximetry sensors for multi-site applications. Design for neonates to adults. Flexible application. Easy to clean.
EnviteC by Honeywell
Others
- Industry
- Healthcare
Others
- Industry
- Healthcare
- Industry : Healthcare
Please sign in to access more documents.
Once signed in, you may be able to access additional documents for your account.
Brochure
Certificate
Others
Please sign in to view part numbers available for purchase based on your account Sign In