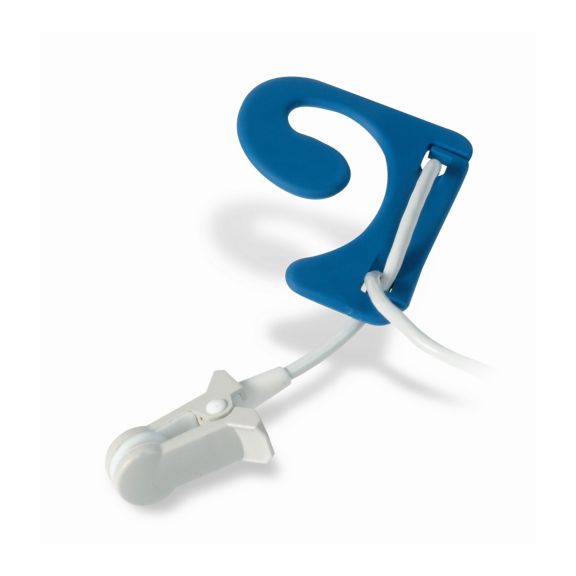
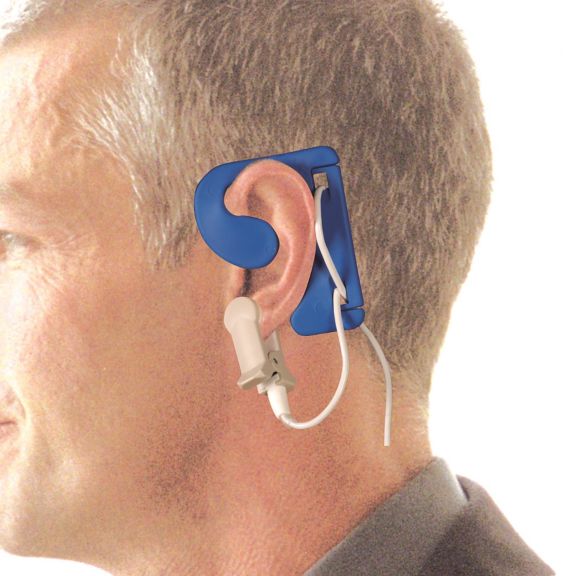
pulse oximetry sensors
Ear Sensors
EarClip reusable pulse oximetry sensors deliver patient comfort – lightweight and free-moving space. Workflow optimization – easy-to-apply and stays in place. High reliability – fast response and less motion artifacts.
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Using a SpO2 probe for non-invasive monitoring of arterial oxygen saturation is a standard of care in most hospital settings and other medical environments.
The most commonly-used probes are placed on the fingertip of the patient. But there are other ways of probe positioning that provide good clinical practice and offer alternatives, especially when the conditions are bad.
The EarClip Sensor is a proven and clinically utilized measuring point. Especially with regard to clinical accuracy, the EarClip is not only equivalent to the FingerClip, but in some cases has even been reported to be superior.
For children and adults, the ear lobe may be the preferred measuring point due to reduced interference from motion artifacts.
EnviteC by Honeywell
Others
- Industry
- Healthcare
Others
- Industry
- Healthcare
- Industry : Healthcare
Please sign in to access more documents.
Once signed in, you may be able to access additional documents for your account.
Brochure
Certificate
Others
Please sign in to view part numbers available for purchase based on your account Sign In