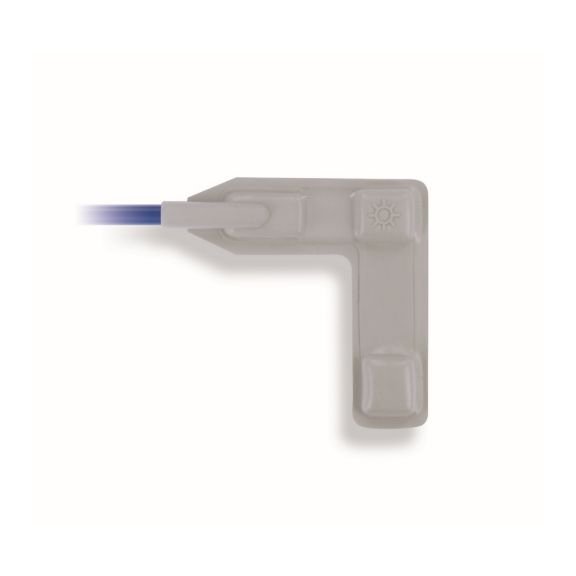
pulse oximetry sensors
Wrap Sensors
Reusable wrap pulse oximetry sensors for neonates to adults. Flexible application. Easy to clean
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Reusable wrap pulse oximetry sensors that wrap. Easy to clean and offer flexible applications
EnviteC by Honeywell
Por favor, faça login para acessar documentos adicionais.
Após o login, você terá acesso a documentos adicionais vinculados à sua conta.
Certificado
Others
Por favor faça login para ver os números de peças disponíveis para compra com base na sua conta Entrar
Filters
Por favor faça login para ver os números de peças disponíveis para compra com base na sua conta Entrar