pulse oximetry sensors
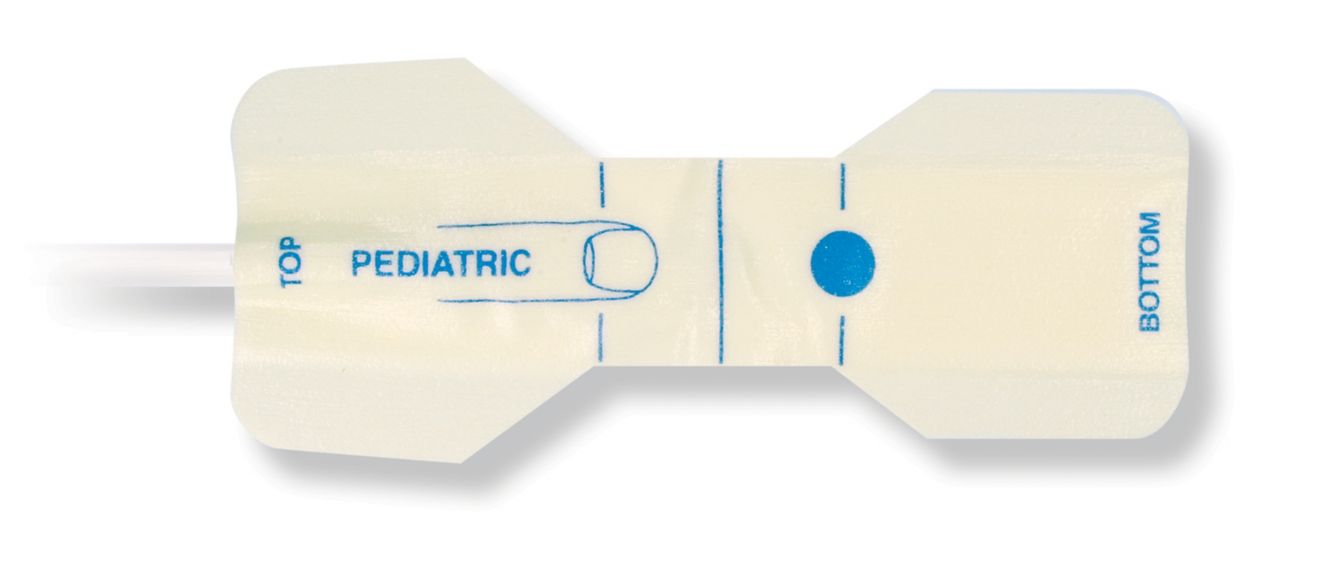
Pediatric Disposable Pulse Oximetry Sensors
Disposable pulse oximetry sensors for children. Comfort in re-positioning (soft medical grade tape). Less adhesive-related skin trauma where necessary – neonates, geriatric patients, patients with burn injuries and adapted for environment
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Disposable SpO2 sensors for children. These sensors do not entail the risk of cross-contamination caused by medical products that are re-used from patient to patient.
The high flexibility, adaptability and bio-compatibility of the applied soft material makes this sensor type ideal for applications where the patient needs extended long-term monitoring.
EnviteC by Honeywell
Por favor, faça login para acessar documentos adicionais.
Após o login, você terá acesso a documentos adicionais vinculados à sua conta.
Certificado
Others
Instructions for Use
Por favor faça login para ver os números de peças disponíveis para compra com base na sua conta Entrar
Filters
Por favor faça login para ver os números de peças disponíveis para compra com base na sua conta Entrar