pulse oximetry sensors
맥박 산소측정기 및 악세사리
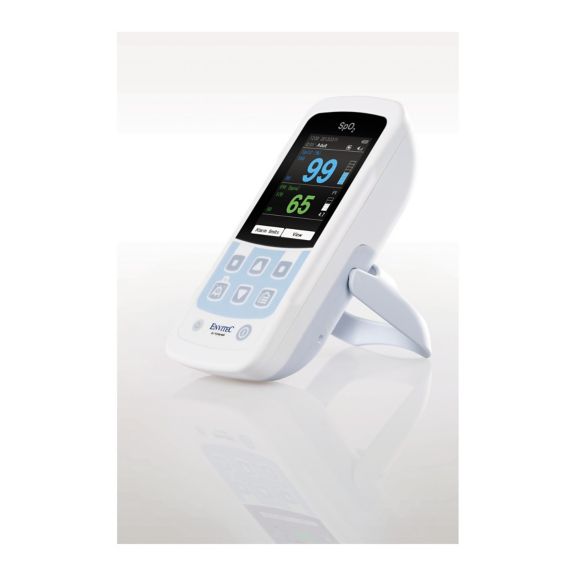
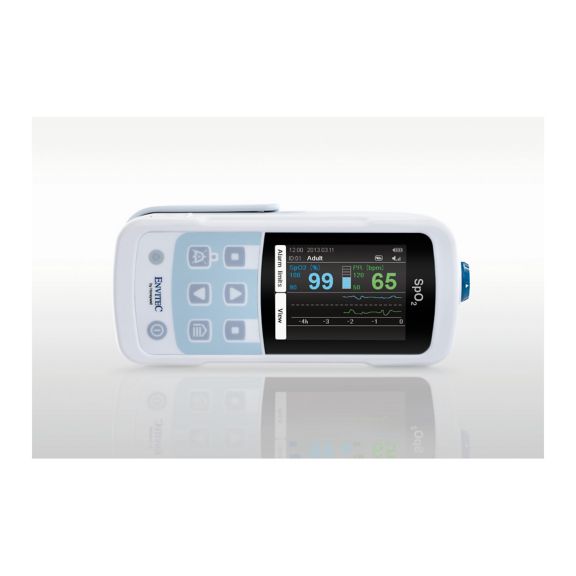
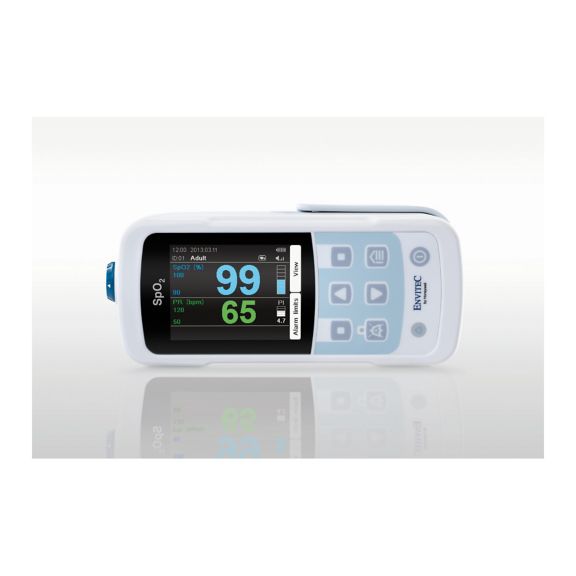
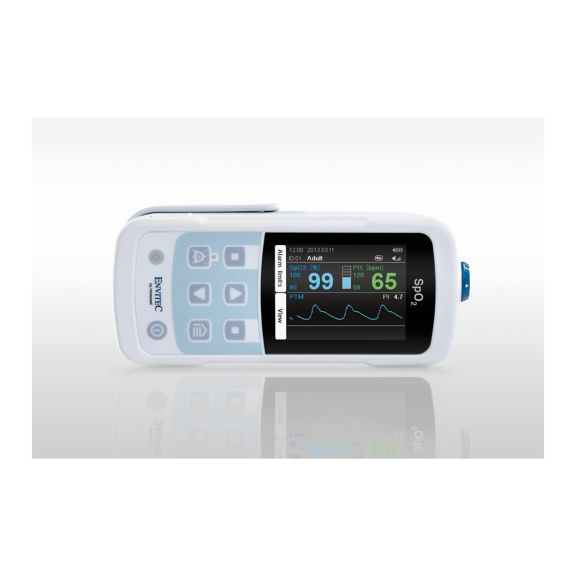
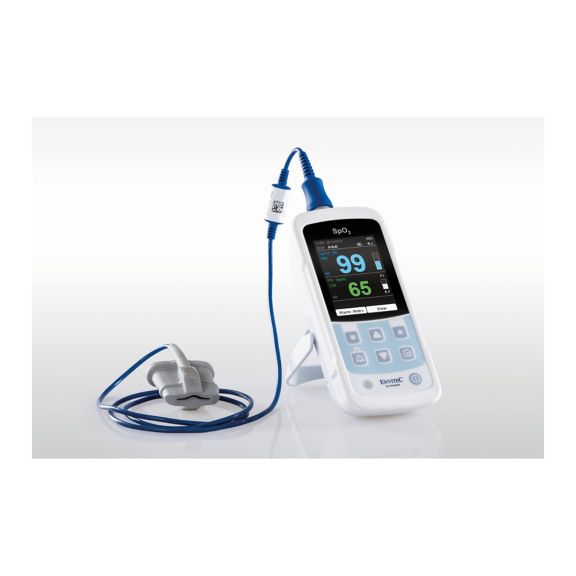
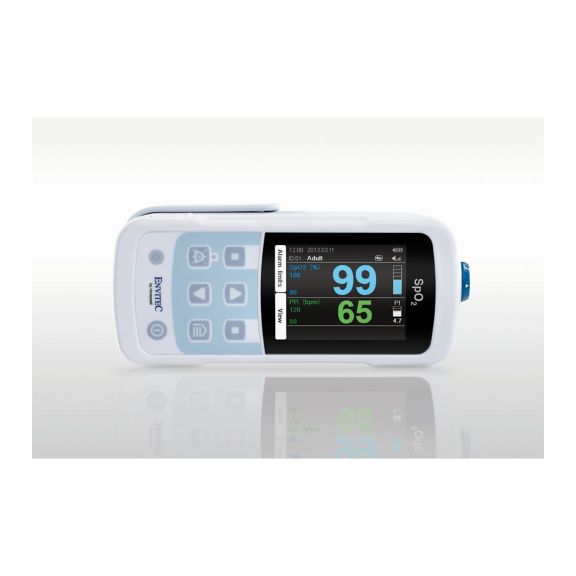
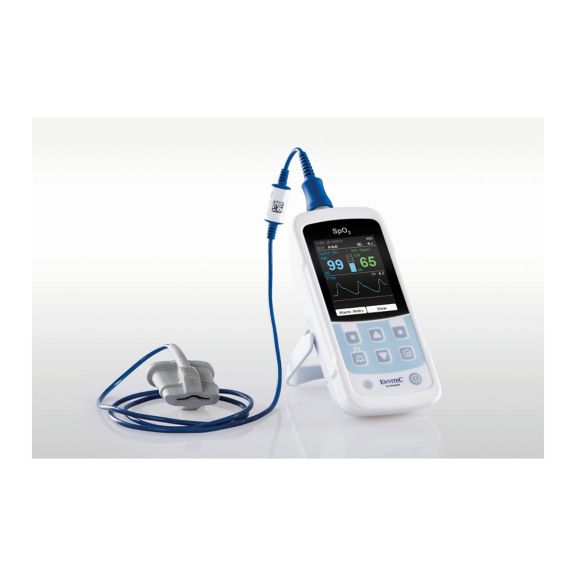
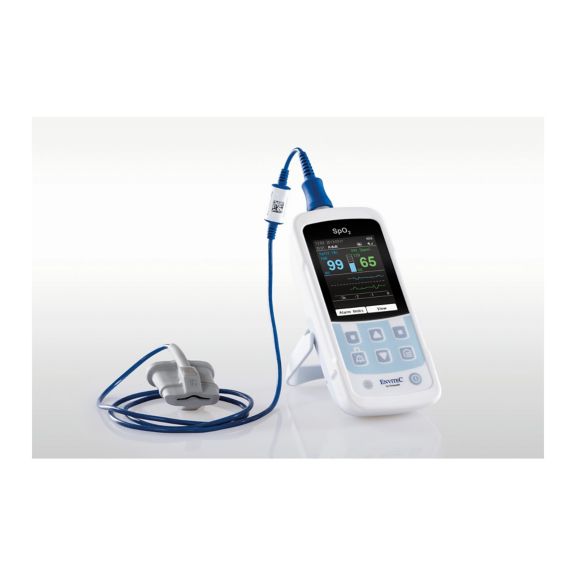
하나의 맥박 산소측정기 장치 – 많은 옵션. 연속 및 순간 점검 SpO2 모니터링에 적합합니다. 휴대용 또는 고정 장치로 설계되었습니다. SpO2, 맥박수를 측정하고 체적변동기록 펄스파를 표시합니다.
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
맥박 산소측정은 동맥혈의 기능적 산소 포화도(SpO2)와 맥박 빈도를 측정하는 비침습적이고 간단하며 신뢰할 수 있는 기술입니다.
소형, 이동 가능 및 휴대용 펄스 옥시미터 모니터의 가용성은 의료 환경에서 수많은 다양한 응용 분야 영역을 제공합니다. SpO2 센서는 현업에서의 핵심 요소입니다. 이는 환자의 해당 신체 부위에 적용됩니다.
MySign® S는 비침습적 활력 징후 모니터링을 위한 지능형 솔루션으로서, 매우 정확한 판독을 제공하는 사용하기 쉬운 모니터입니다.
Honeywell의 EnviteC
더 많은 문서에 접근하시려면 로그인해 주시기 바랍니다.
로그인하시면계정과 관련된 추가 문서에 접근하실 수 있습니다.
브로슈어
인증서
Others
제발 계정에 따라 구매 가능한 부품 번호를 보려면 로그인하세요. 로그인