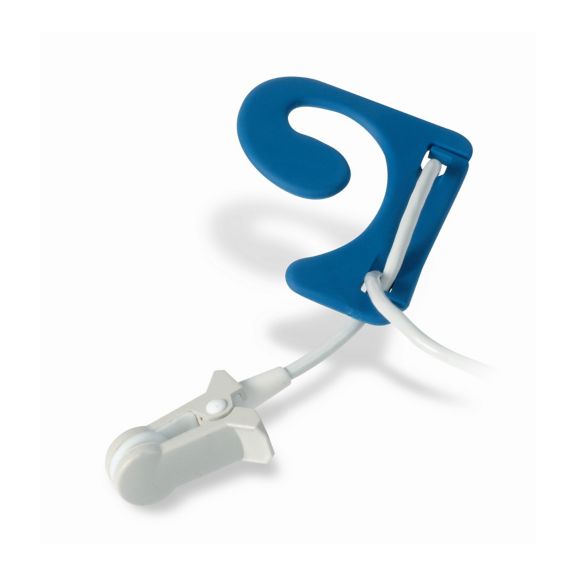
pulse oximetry sensors
이어 센서:
재사용이 가능한 EarClip 맥박 산소측정 센서는 환자에게 가볍고 자유로운 이동 공간을 제공합니다. 워크플로우 최적화 – 적용이 쉽고 제자리에 유지됩니다. 높은 신뢰성 – 빠른 응답 및 모션 왜곡 감소
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
동맥 산소 포화도의 비침습적 모니터링에 SpO2 프로브를 사용하는 것은 대부분의 병원 환경 및 기타 의료 환경에서 표준 치료입니다.
가장 일반적으로 사용되는 프로브는 환자의 손가락 끝에 위치합니다. 그러나 좋은 임상 관행을 제공하고 특히 상태가 좋지 않은 경우 대안을 제공하는 프로브 포지셔닝의 다른 방법들이 있습니다.
EarClip 센서는 입증되고 임상적으로 활용되는 측정 포인트입니다. 특히 임상적 정확성과 관련하여, EarClip은 FingerClip과 동등할 뿐만 아니라 일부의 경우 우월한 것으로 보고되고 있다.
어린이와 성인의 경우, 모션 아티팩트의 간섭 감소로 인해 귓불이 선호되는 측정 지점이 될 수 있습니다.
Honeywell의 EnviteC
더 많은 문서에 접근하시려면 로그인해 주시기 바랍니다.
로그인하시면계정과 관련된 추가 문서에 접근하실 수 있습니다.
브로슈어
인증서
Others
제발 계정에 따라 구매 가능한 부품 번호를 보려면 로그인하세요. 로그인