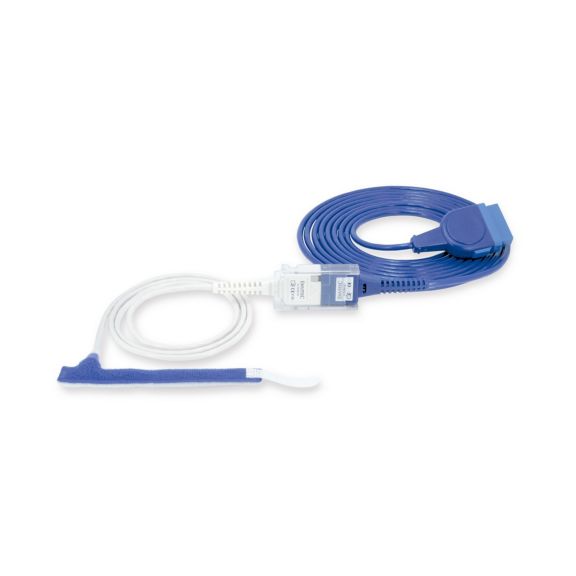
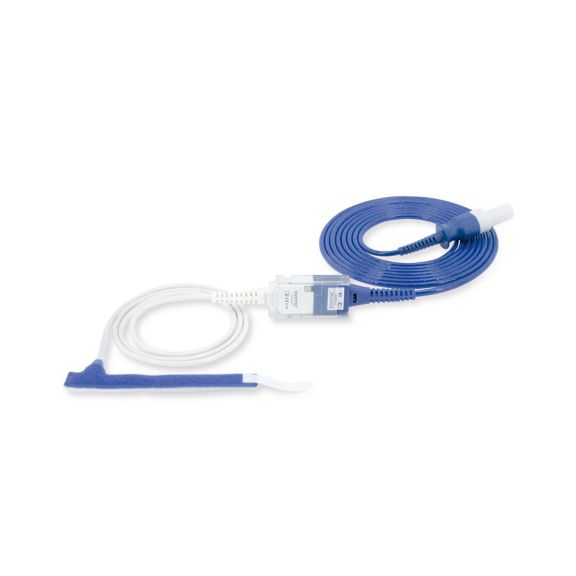
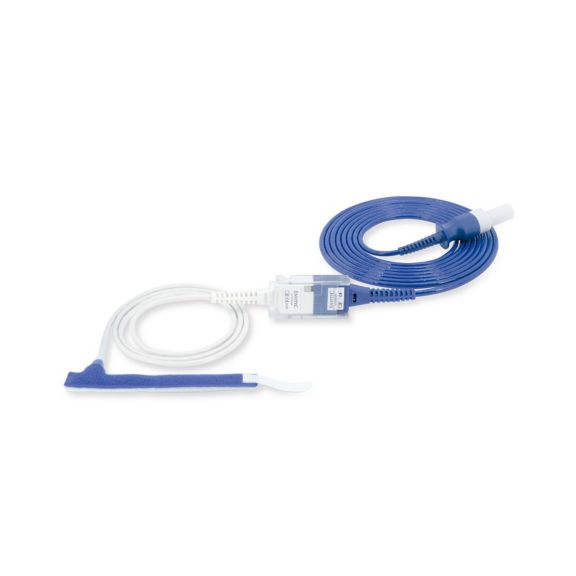
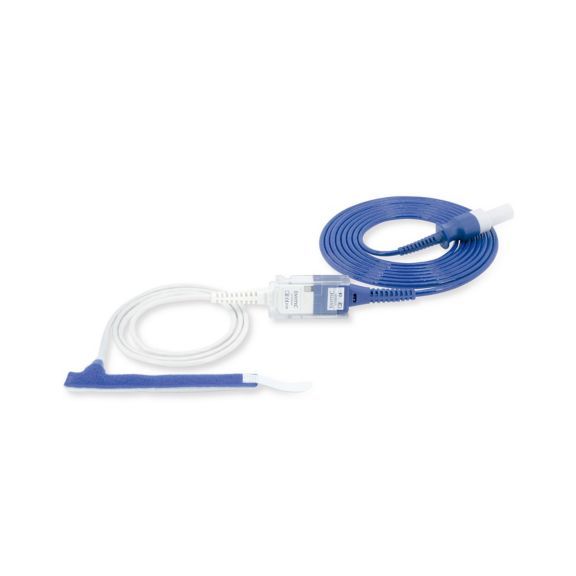
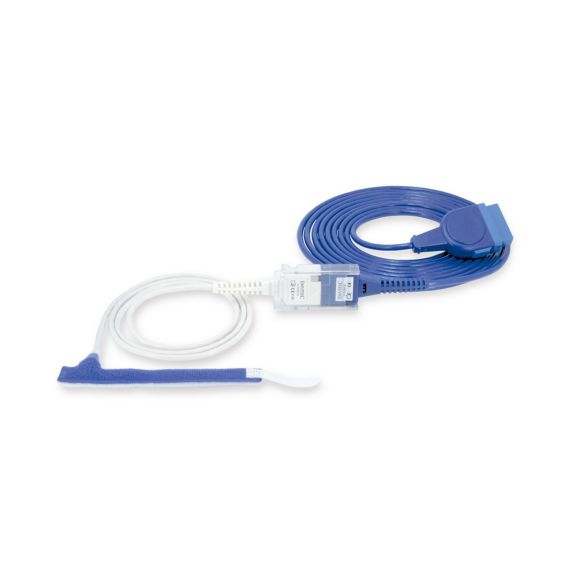
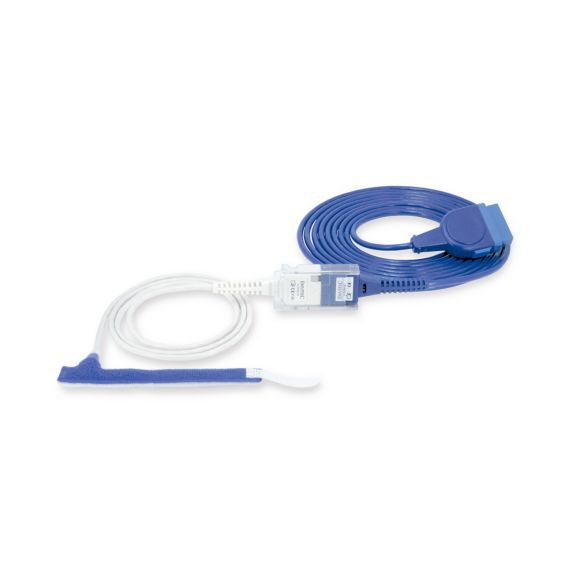
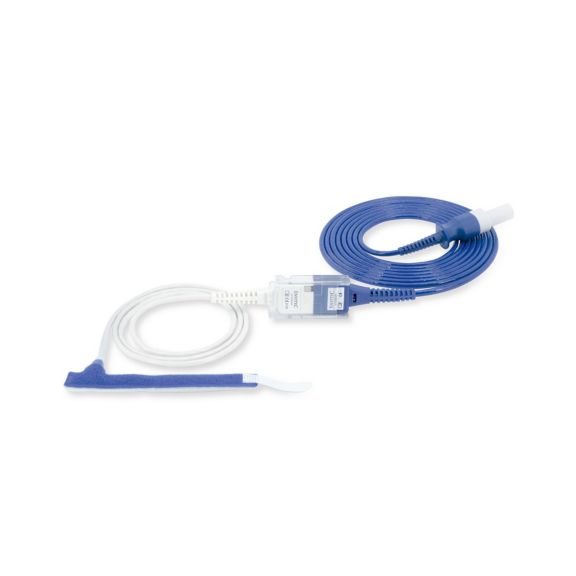
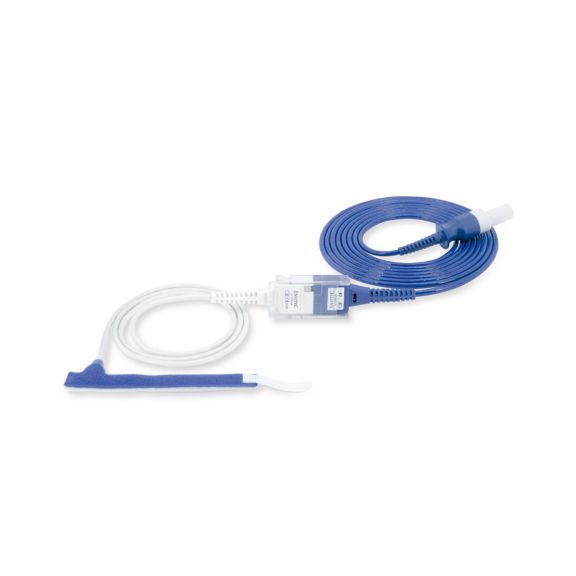
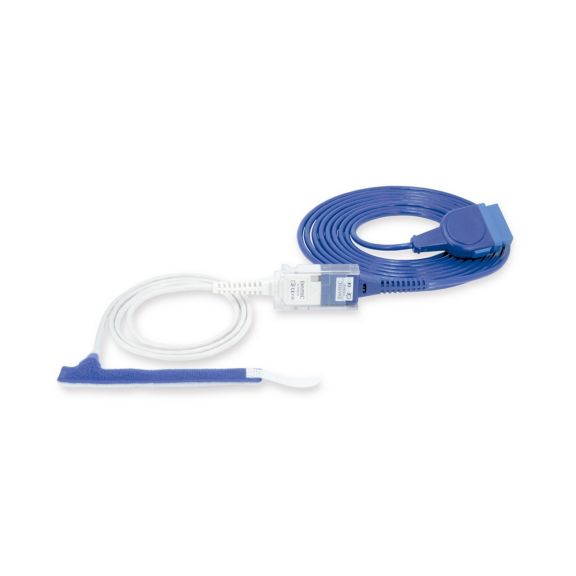
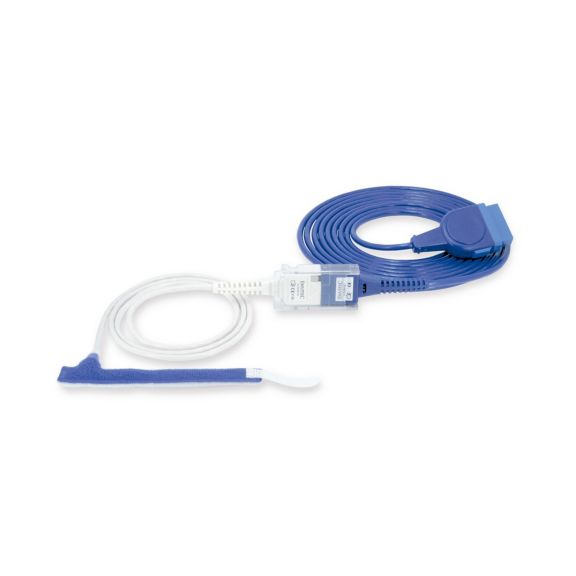
パルスオキシメトリーセンサ
ラップ式
新生児から成人まで使用できる、ラップ式パルスオキシメータ用センサ 柔軟なアプリケーション クリーニングが簡単
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
より多くのドキュメントにアクセスするためには、サインインをしてください。
サインインすることで追加ドキュメントにアクセスできるようになります。
認証
その他
Instructions for Use
お願いします サインインすると、アカウントに基づいて購入可能な部品番号が表示されます サインイン