パルスオキシメトリーセンサ
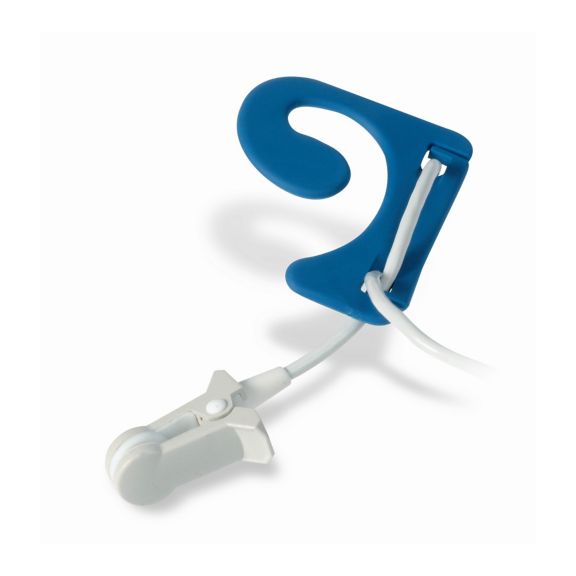
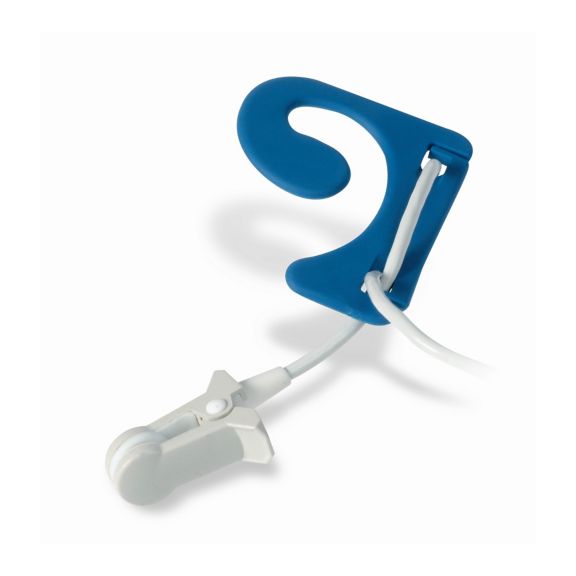
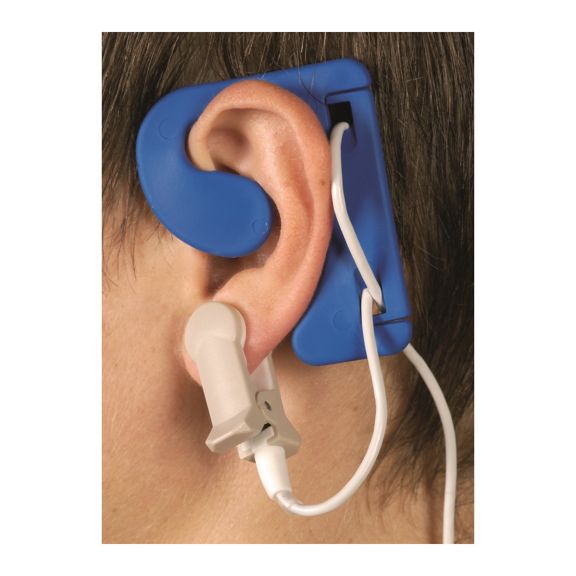
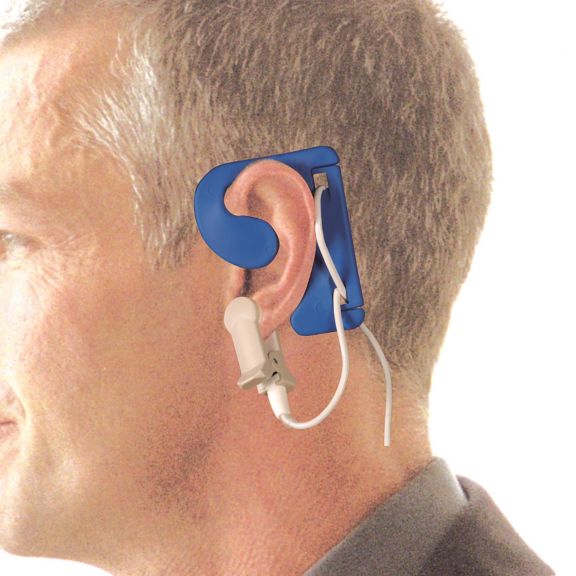
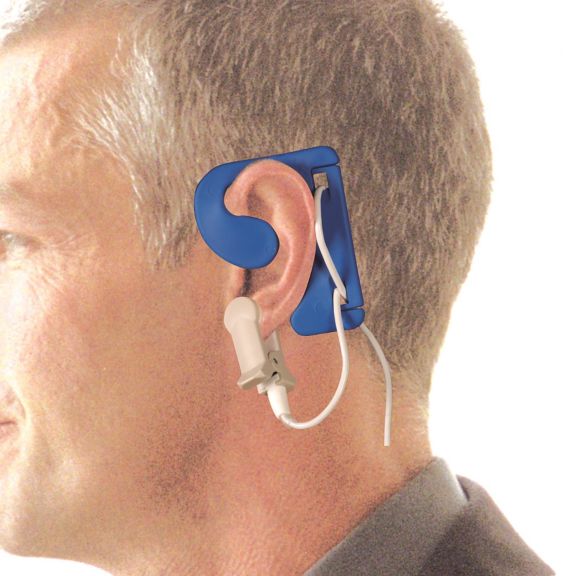
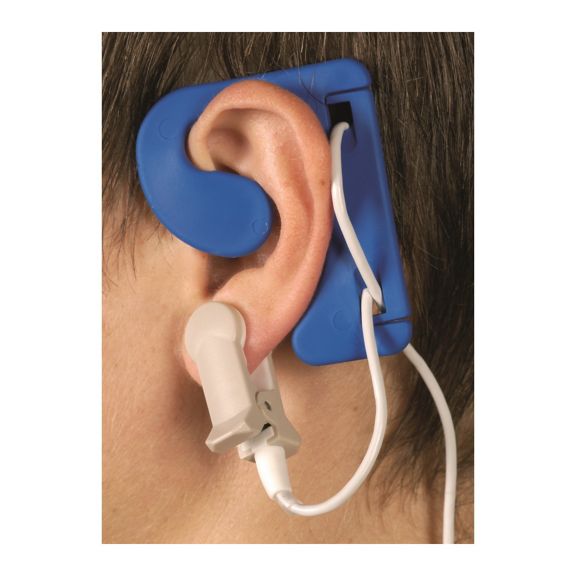
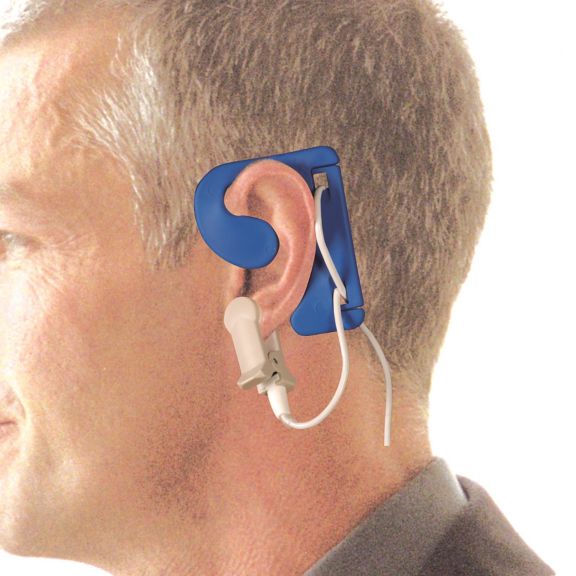
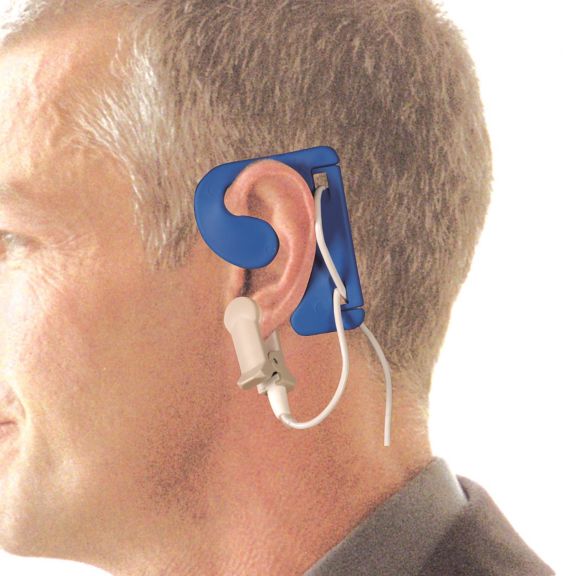
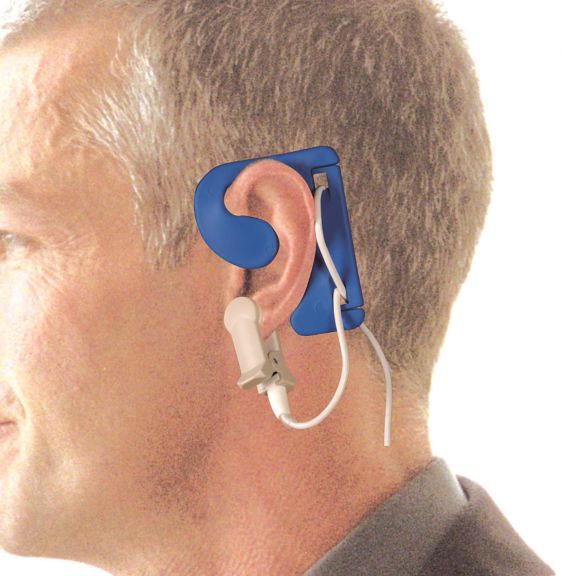
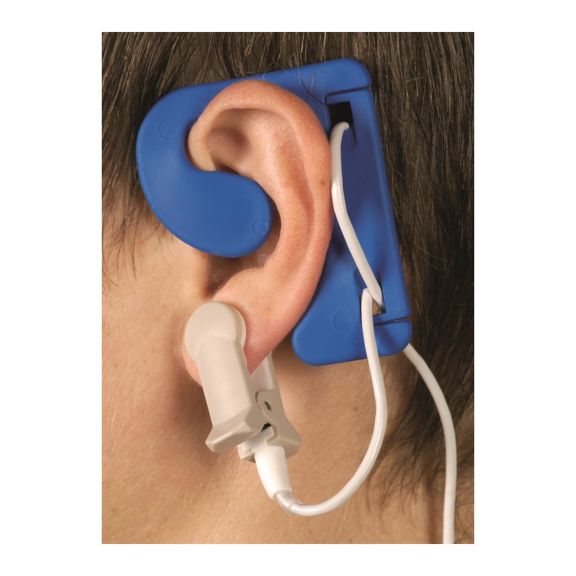
耳用センサ
EarClipリユーザブル パルスオキシメータ用センサは、患者さんに快適な装着感だけでなく、軽量で自由に移動できる空間を提供します。ワークフローの最適化 – 適用が容易で、常に適切な状態に保たれます。高い信頼性 – 迅速な応答と少ないモーションアーチファクト
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
SpO2プローブを使用した動脈血酸素飽和度の非侵襲モニタリングは、ほとんどの病院環境およびその他の医療環境での標準的なケアです。
最も一般的に使用されるプローブは、患者さんの指先に取り付けます。しかし、特に条件が悪い場合には、優れた臨床的手法を提供し、代替手段となる、他の方法によるプローブのポジショニングがあります。
EarClipセンサは、実績があり、臨床で利用されている測定ポイントです。特に臨床精度に関しては、EarClipはFingerClipと同等であるだけでなく、場合によっては優れていると報告されています。
小児および成人の場合、モーションアーチファクトによる干渉が減少するため、耳たぶが望ましい測定ポイントとなることがあります。
ハネウェル製EnviteC
より多くのドキュメントにアクセスするためには、サインインをしてください。
サインインすることで追加ドキュメントにアクセスできるようになります。
ブローシャ―
認証
その他
お願いします サインインすると、アカウントに基づいて購入可能な部品番号が表示されます サインイン