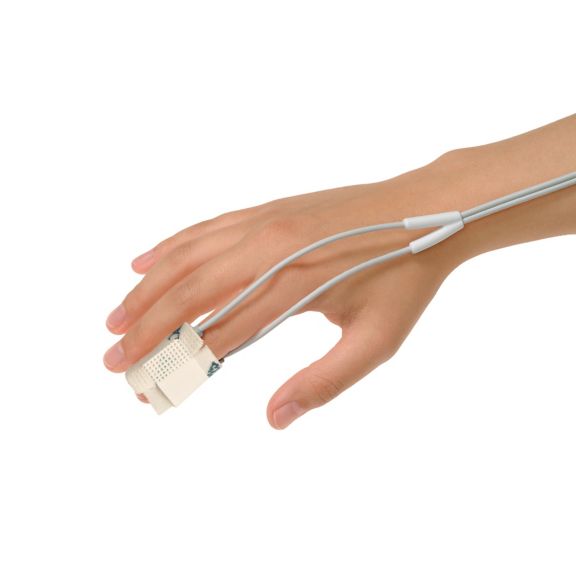
pulse oximetry sensors
Y Sensors
Y reusable pulse oximetry sensors for multi-site applications. Design for neonates to adults. Flexible application. Easy to clean
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Y reusable pulse oximetry sensors for multi-site applications. Design for neonates to adults. Flexible application. Easy to clean.
EnviteC by Honeywell
Veuillez vous identifier pour accéder à plus de documents.
Une fois connecté, vous pourrez accéder à des documents supplémentaires pour votre compte.
Brochure
Certificat
Autres
S'il te plaît connectez-vous pour voir les numéros de pièces disponibles à l'achat en fonction de votre compte Se connecter
Filters
S'il te plaît connectez-vous pour voir les numéros de pièces disponibles à l'achat en fonction de votre compte Se connecter