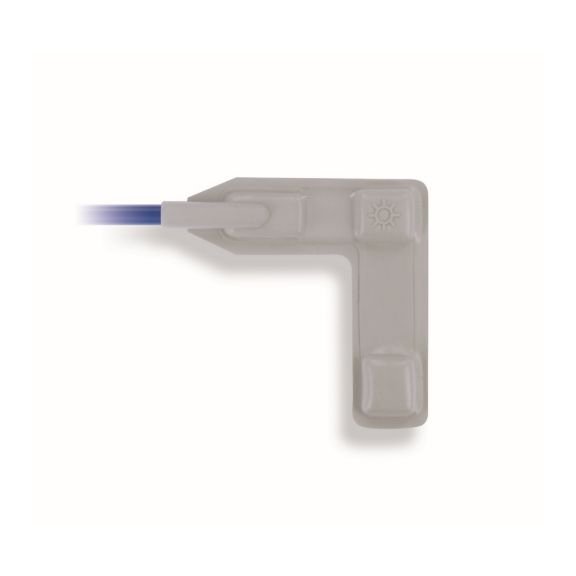
pulse oximetry sensors
Wrap Sensors
Reusable wrap pulse oximetry sensors for neonates to adults. Flexible application. Easy to clean
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Reusable wrap pulse oximetry sensors that wrap. Easy to clean and offer flexible applications
EnviteC by Honeywell
Veuillez vous identifier pour accéder à plus de documents.
Une fois connecté, vous pourrez accéder à des documents supplémentaires pour votre compte.
Certificat
Autres
S'il te plaît connectez-vous pour voir les numéros de pièces disponibles à l'achat en fonction de votre compte Se connecter
Filters
S'il te plaît connectez-vous pour voir les numéros de pièces disponibles à l'achat en fonction de votre compte Se connecter