pulse oximetry sensors
Wrap
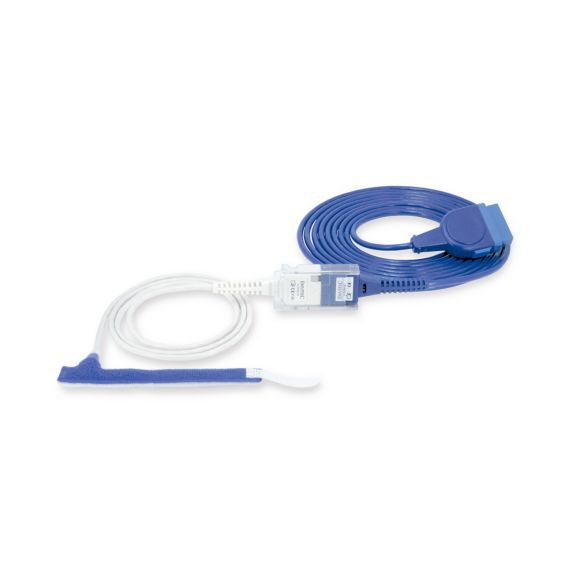
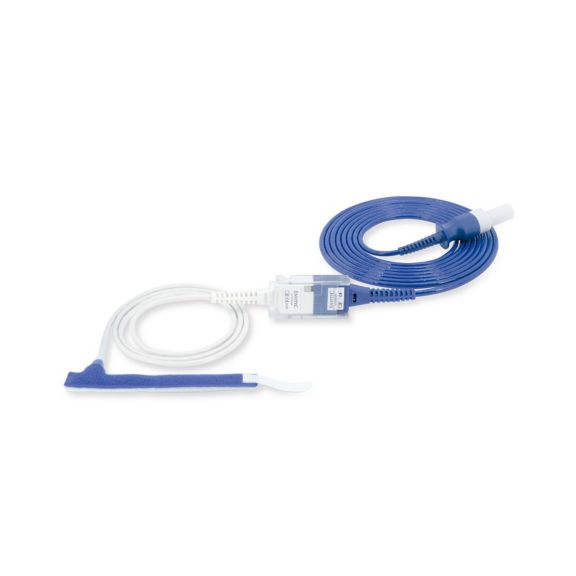
Reuseable wrap pulse oximetry sensors for neonates to adults. Flexible application. Easy to clean
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC Cross Reference List | Tech Support
Resusable wrap pulse oximetry sensors. Easy to clean and flexible applications.
EnviteC by Honeywell
Accedi per visualizzare piu documenti.
Una volta effettuato l’accesso, potresti avere la possibilità di accedere a documenti aggiuntivi relativi al tuo account.
Certificato
Altro
Instructions for Use
Per favore accedi per visualizzare i codici dei componenti disponibili per l'acquisto in base al tuo account Registrazione
Filters
Per favore accedi per visualizzare i codici dei componenti disponibili per l'acquisto in base al tuo account Registrazione