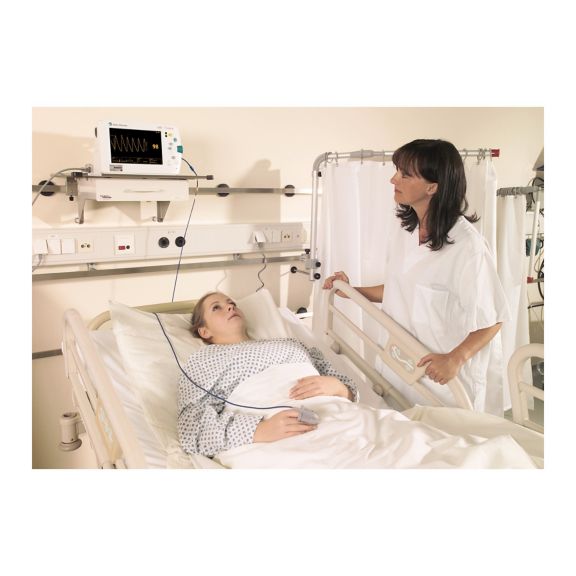
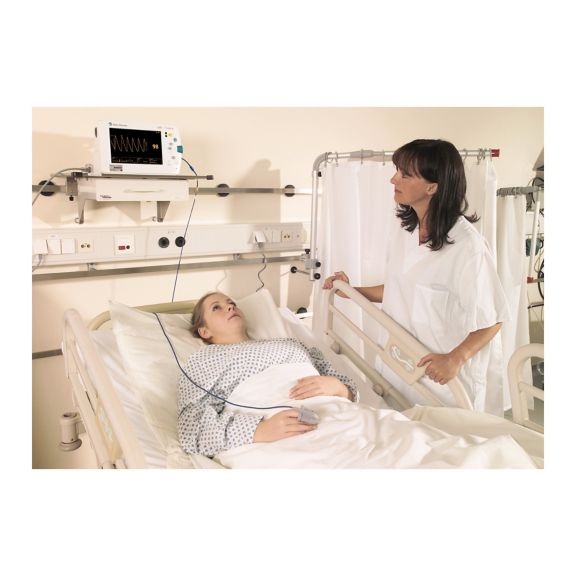
Capteurs d'oxymétrie de pouls
SoftTip®
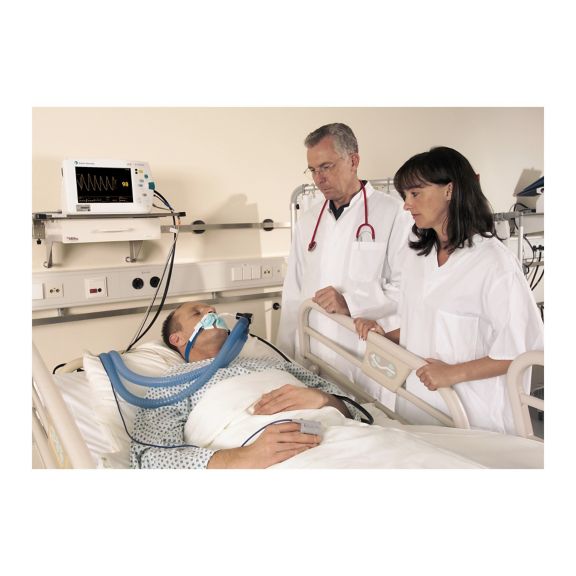
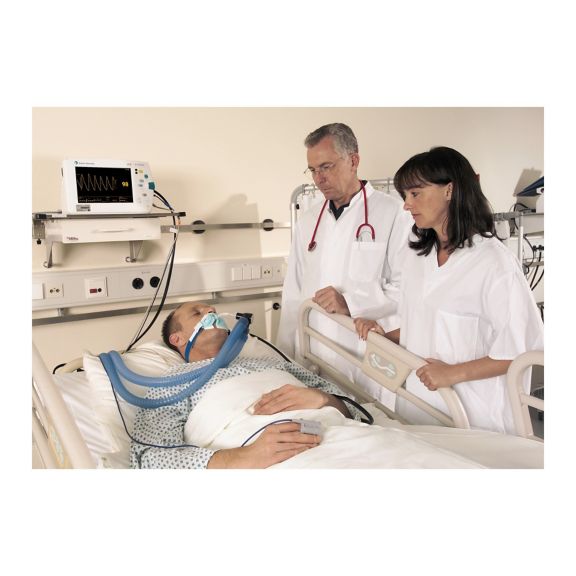
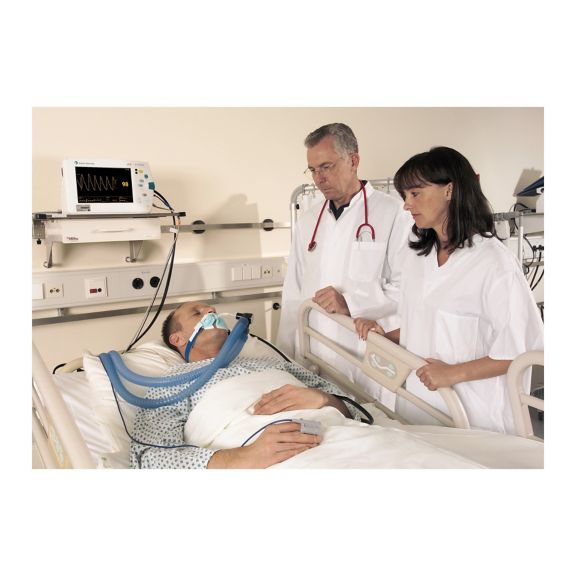
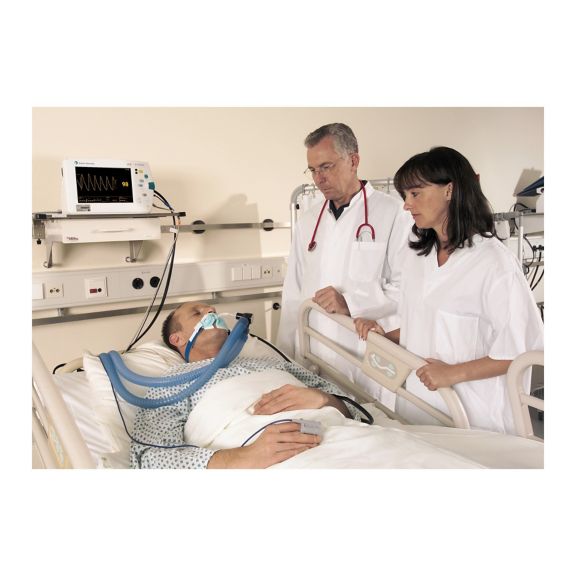
Capteurs d'oxymétrie de pouls réutilisables SoftTip®, faciles à manipuler et à nettoyer. Résistants aux contraintes mécaniques. Offrent un confort optimal avec différentes tailles, adaptés à toutes les applications.
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
Un capteur réutilisable à long terme permettant un nettoyage facile par submersion complète dans un liquide de nettoyage. Résiste également à des contraintes mécaniques maximales. La conception unique du produit garantit un confort extraordinairement élevé pour le patient et des résultats de mesure d’une précision exceptionnelle. La compatibilité avec les dispositifs de surveillance courants de la plupart des fabricants connus est garantie.
EnviteC by Honeywell
Brochure
Certificat
Autres
S'il te plaît connectez-vous pour voir les numéros de pièces disponibles à l'achat en fonction de votre compte Se connecter
Filters
S'il te plaît connectez-vous pour voir les numéros de pièces disponibles à l'achat en fonction de votre compte Se connecter