Pulse Oximetry Sensors
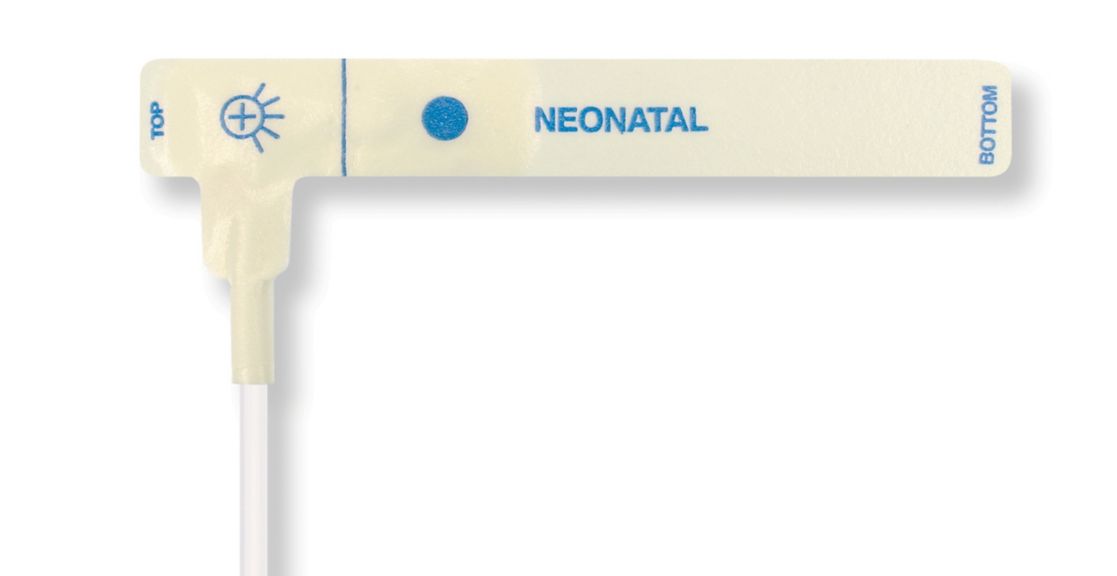
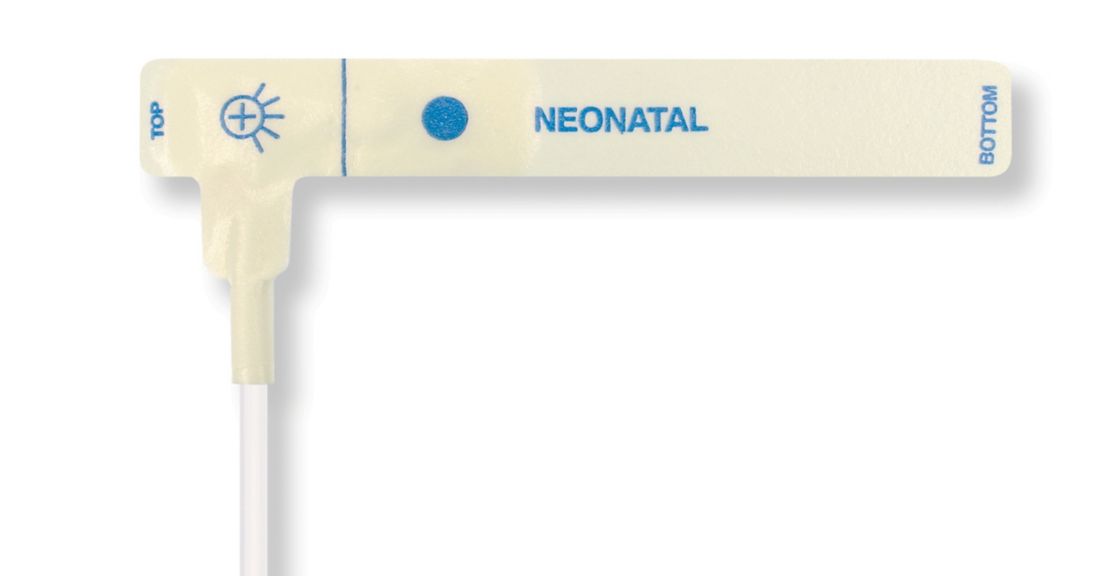
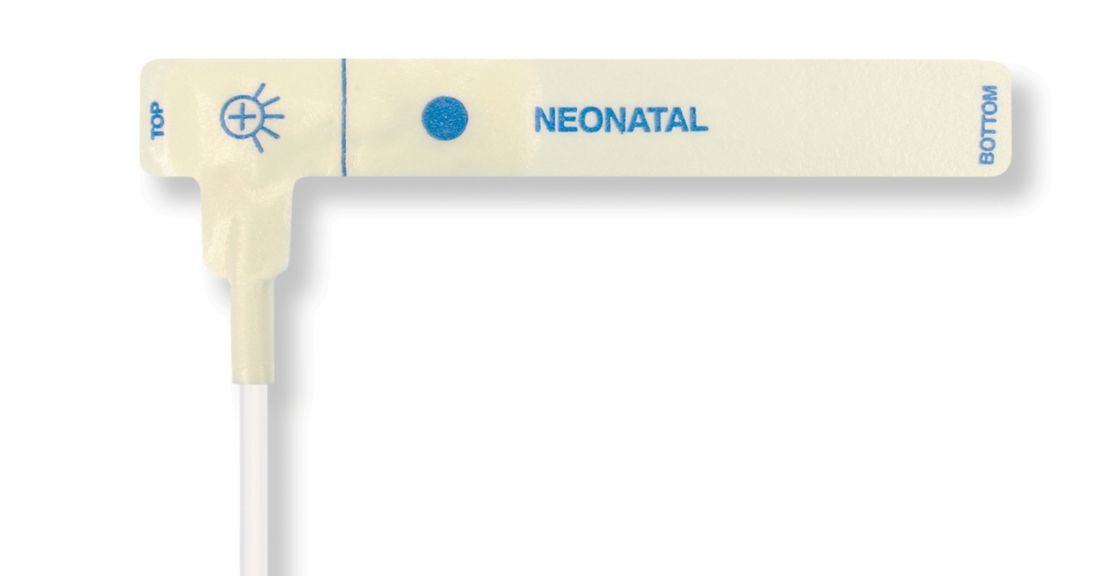
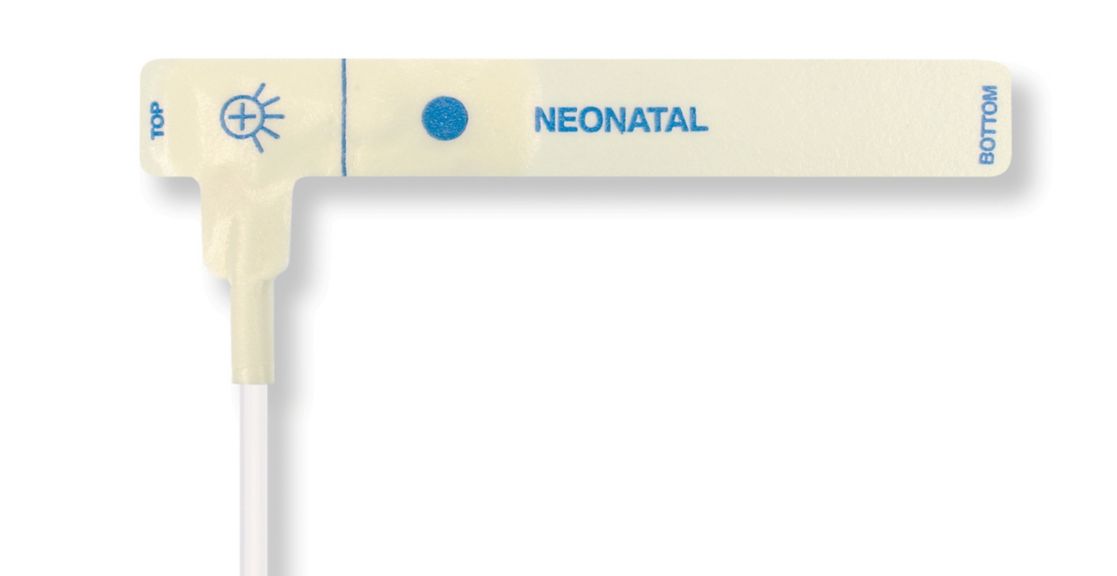
Disposable Pulse Oximetry Sensors for Neonates
• Good fit • Flexible and adaptable • Easy to use
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
Others
- Industry
- Healthcare
Others
- Industry
- Healthcare
- Industry : Healthcare
Certificate
Others
Instructions for Use
Please sign in to view part numbers available for purchase based on your account Sign In