Sensores de oximetría de pulso
Envoltura
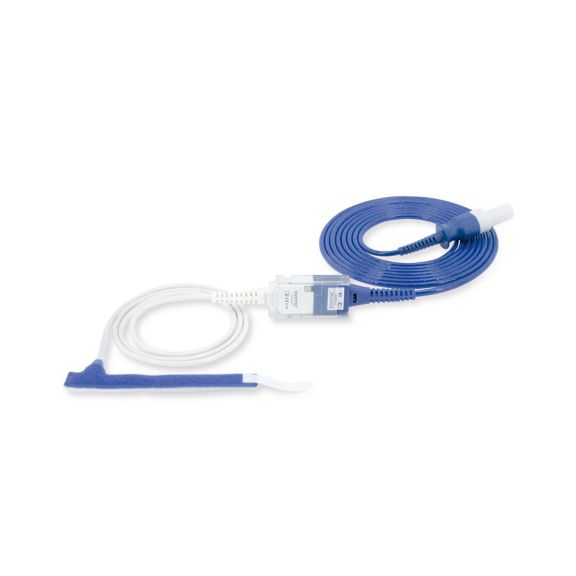
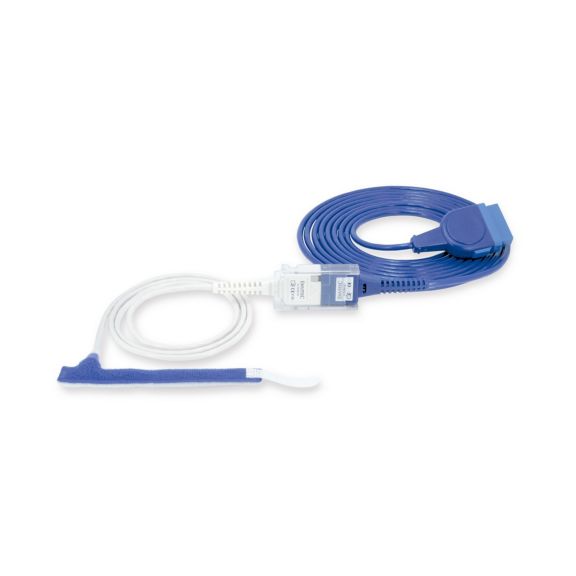
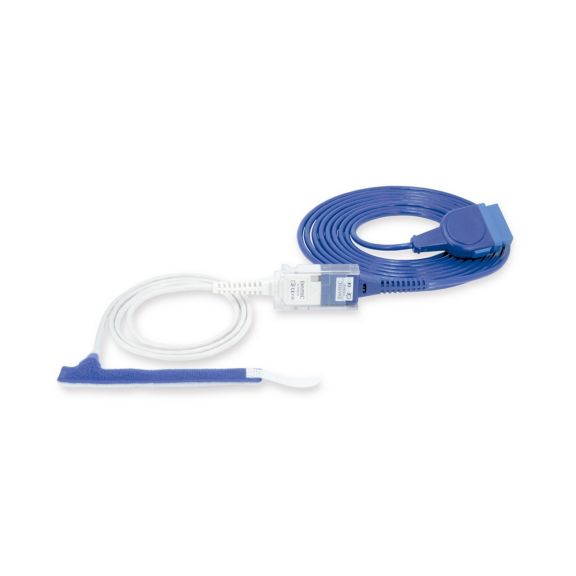
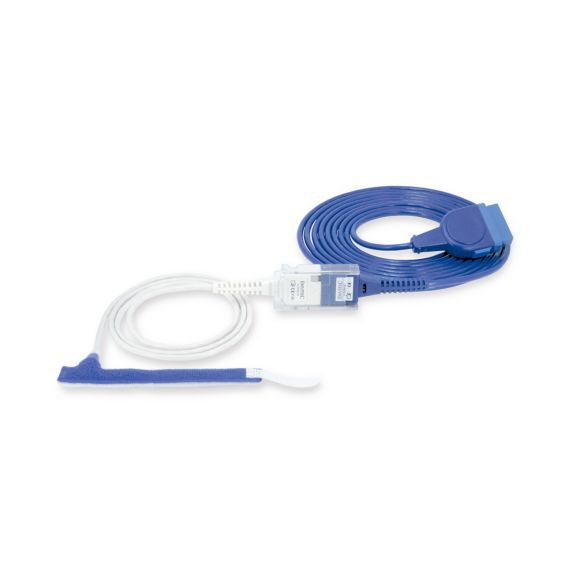
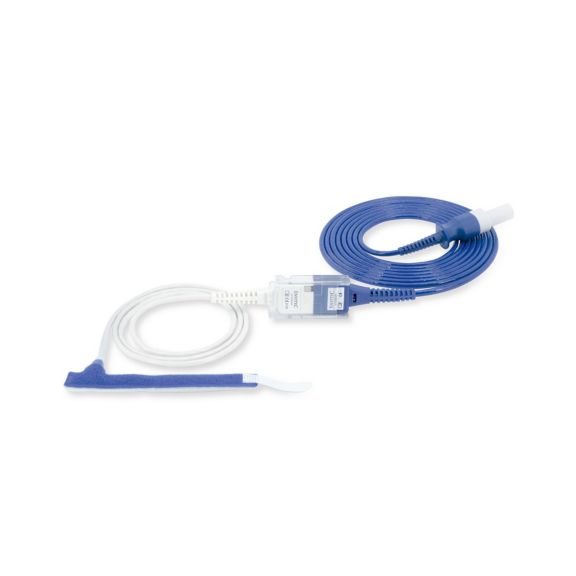
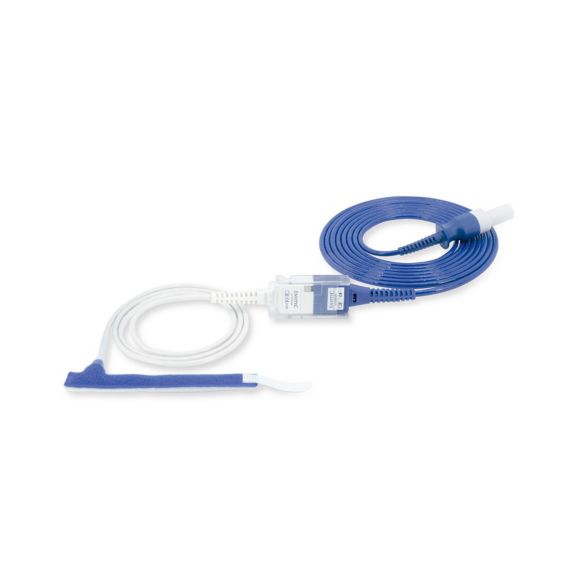
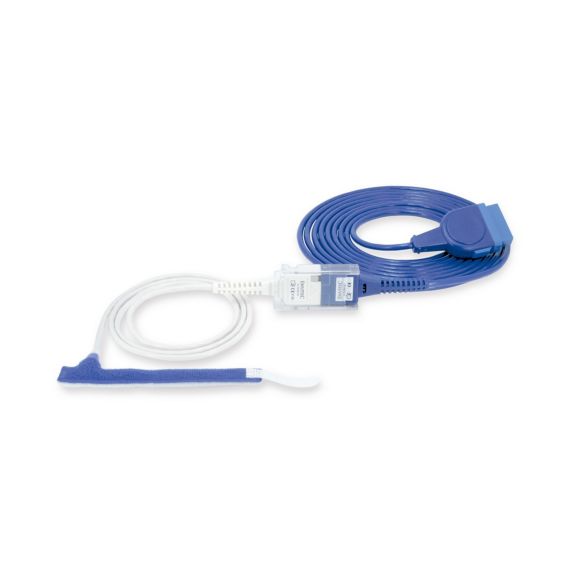
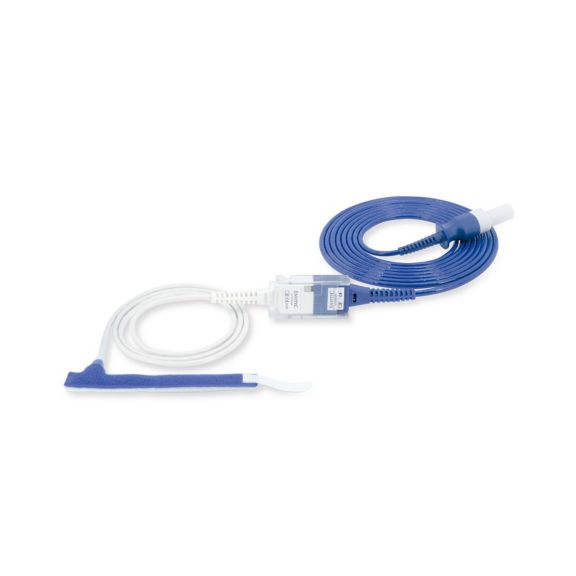
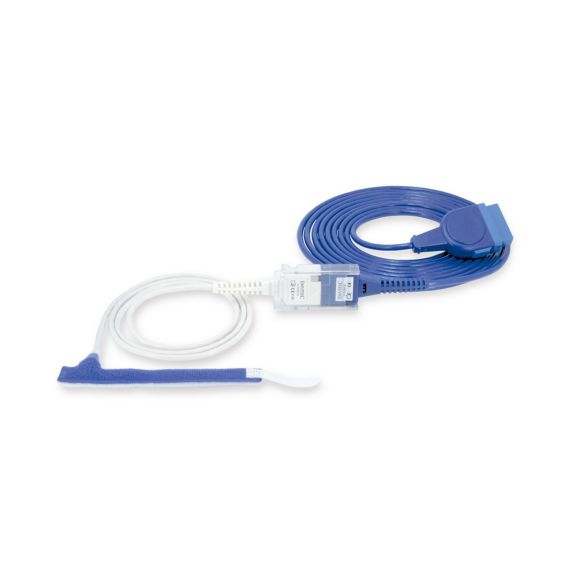
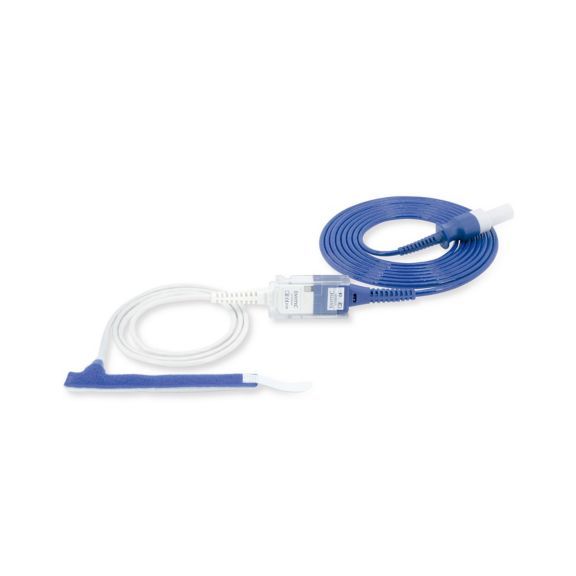
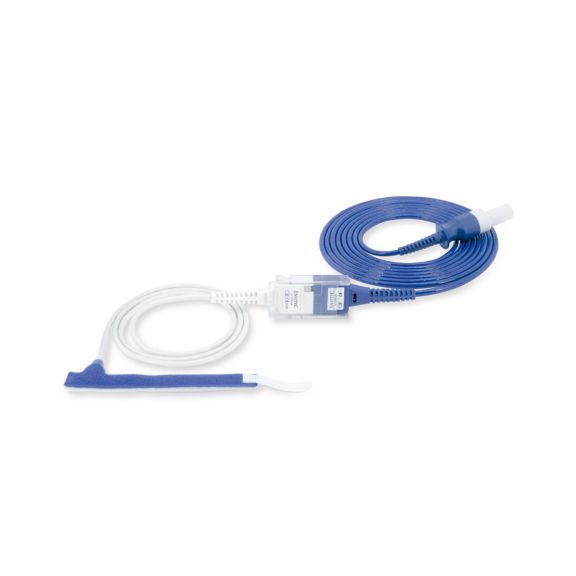
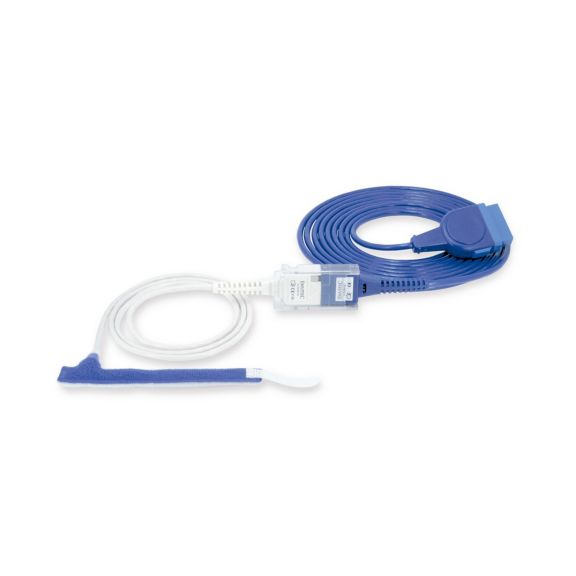
Sensores de pulsioximetría de envoltura reutilizables para neonatos a adultos. Aplicación flexible. Fácil de limpiar
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
Lista de referencias cruzadas de EnviteC | Soporte técnico
Sensores de pulsioximetría de envoltura reutilizables. Aplicaciones fáciles de limpiar y flexibles.
EnviteC by Honeywell
Certificado
Otros
Instructions for Use
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión
Filters
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión