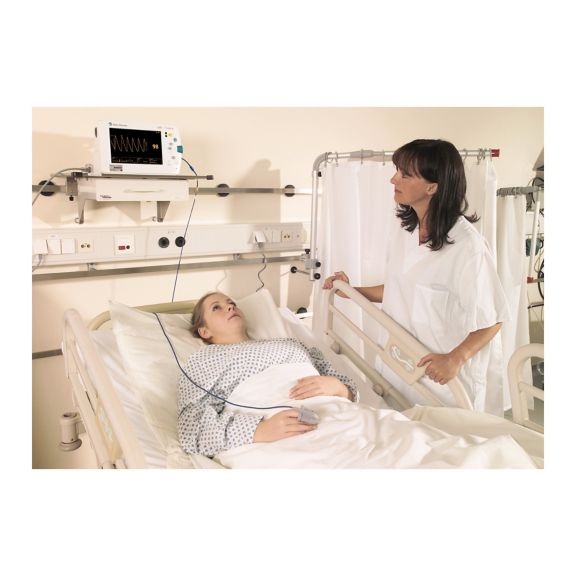
Sensores de oximetría de pulso
SoftTip®
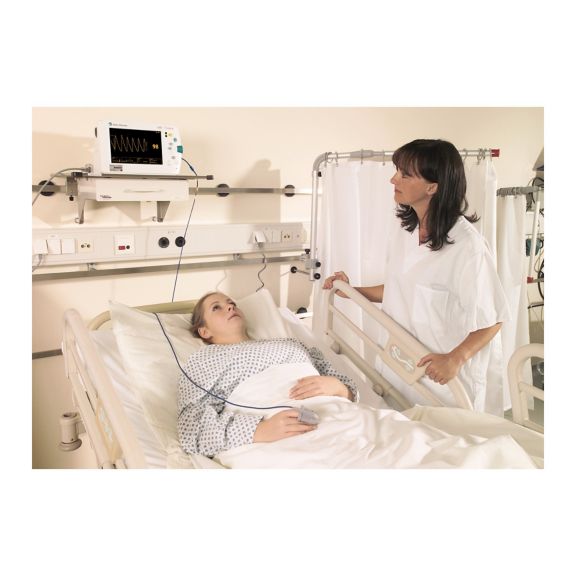
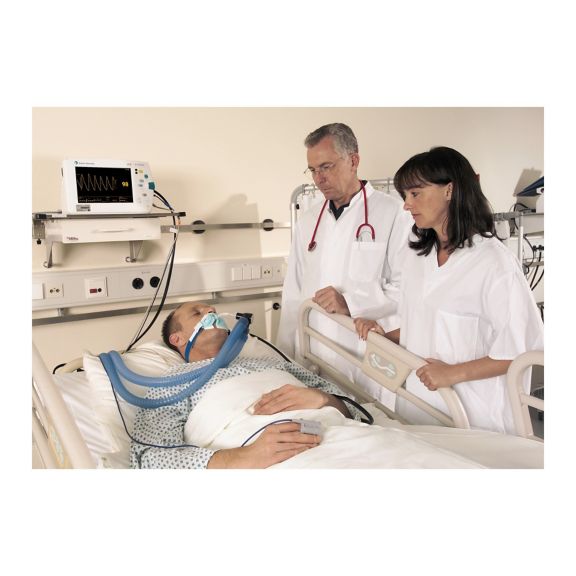
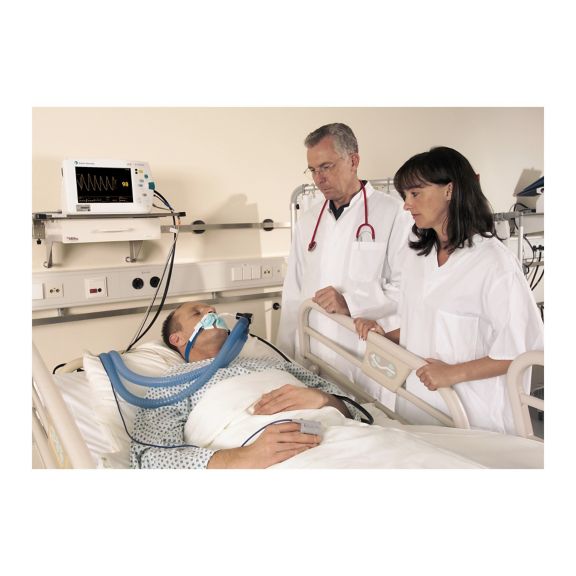
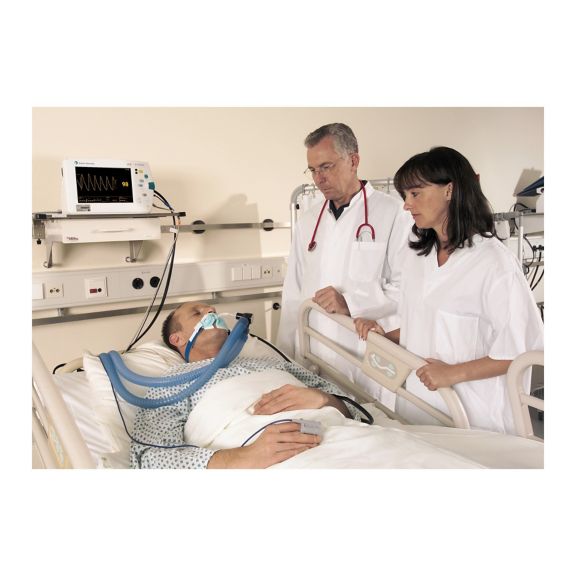
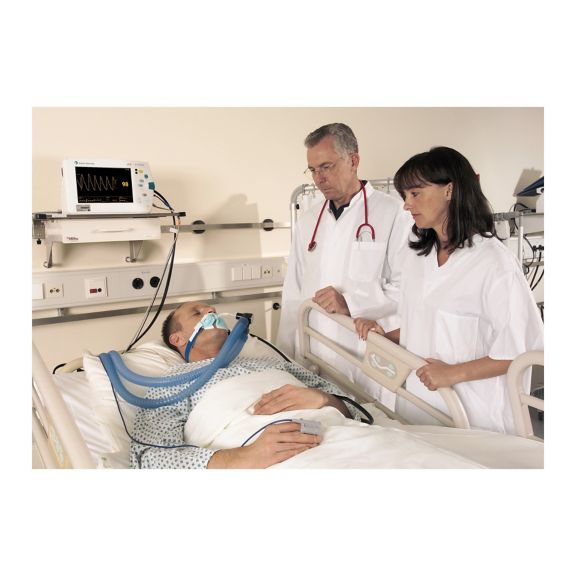
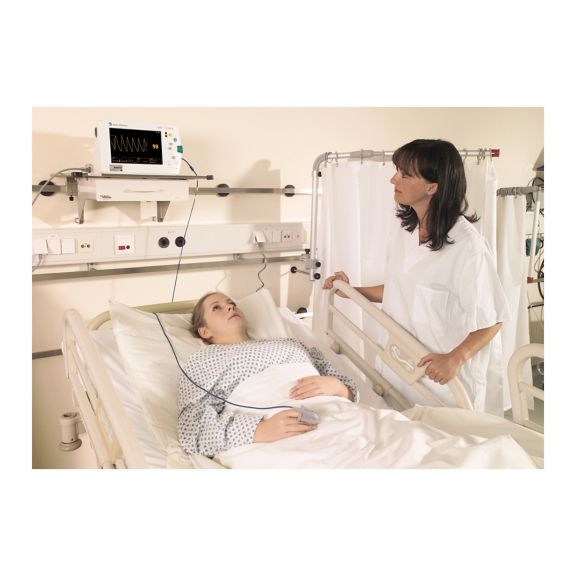
Sensores de pulsioximetría reutilizables SoftTip® con fácil manejo, limpieza óptima y desinfección exhaustiva. Máxima resistencia al estrés mecánico. Gran comodidad de uso gracias a los diversos tamaños para todas las aplicaciones
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
Un sensor del dedo reutilizable a largo plazo que proporciona una limpieza fácil por inmersión completa en líquido limpiador. Además, soporta el máximo estrés mecánico.
El diseño único del producto garantiza una comodidad del paciente extraordinariamente alta y resultados de medición excepcionalmente precisos. Se garantiza la compatibilidad con los dispositivos de monitorización habituales de la mayoría de fabricantes conocidos.
EnviteC by Honeywell
Folleto
Certificado
Otros
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión
Filters
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión