Sensores de oximetría de pulso
Sensores de oído
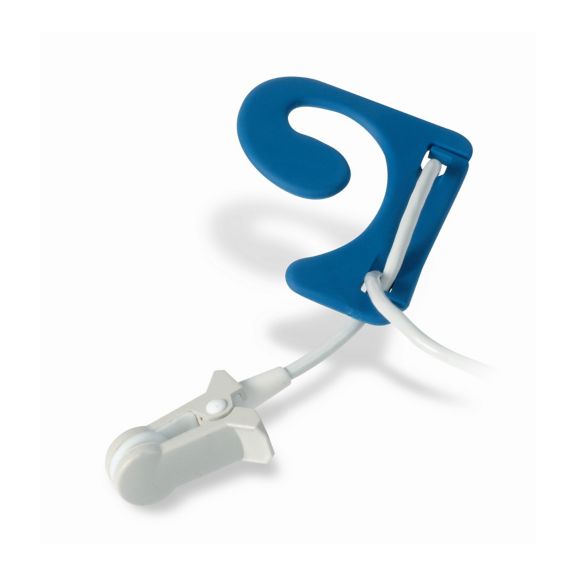
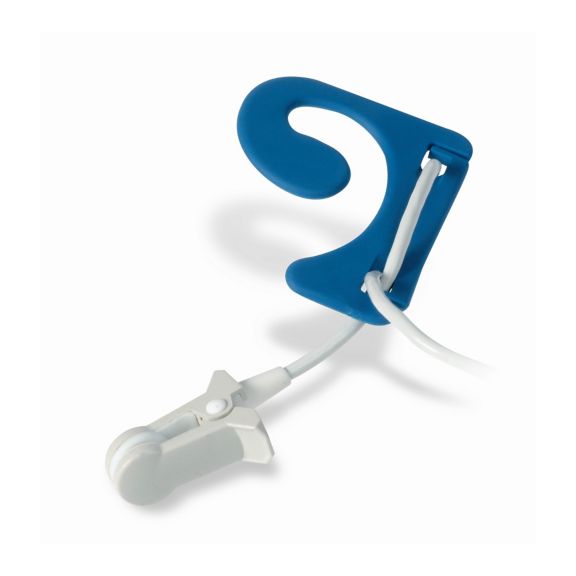
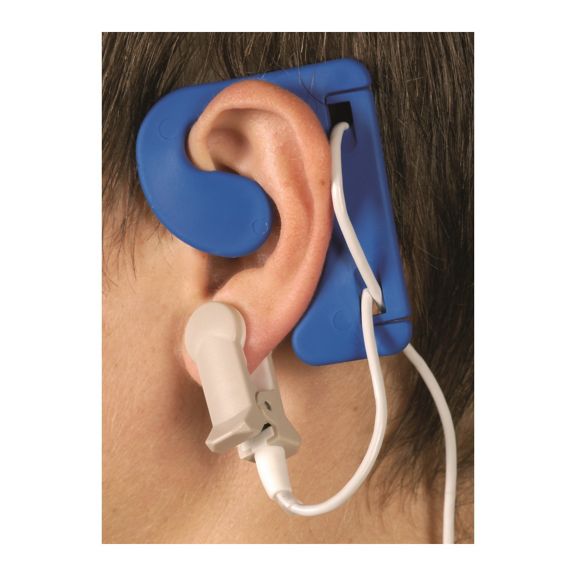
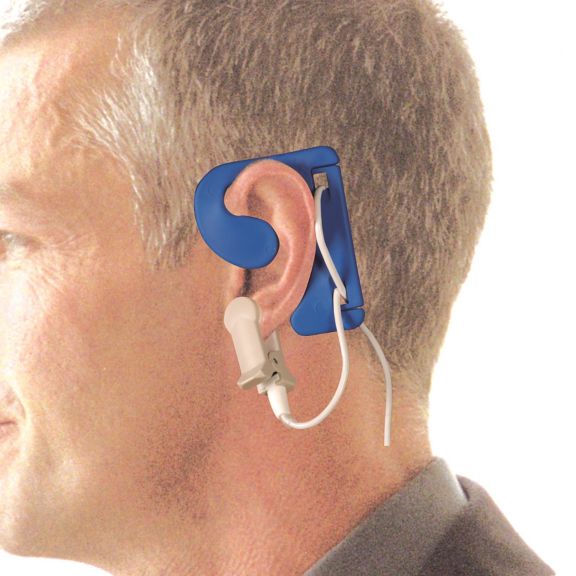
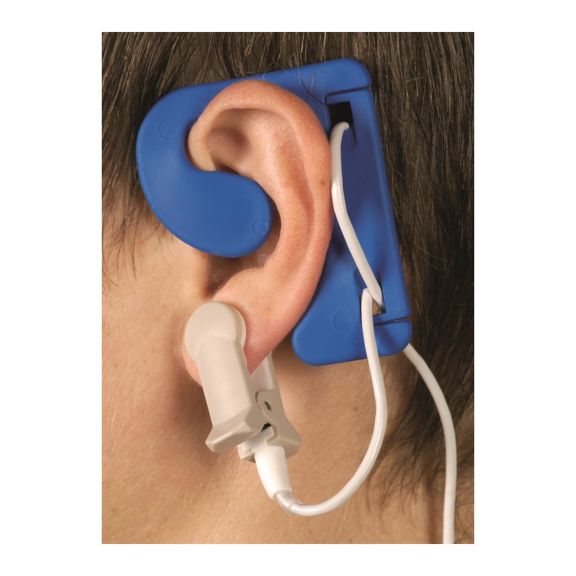
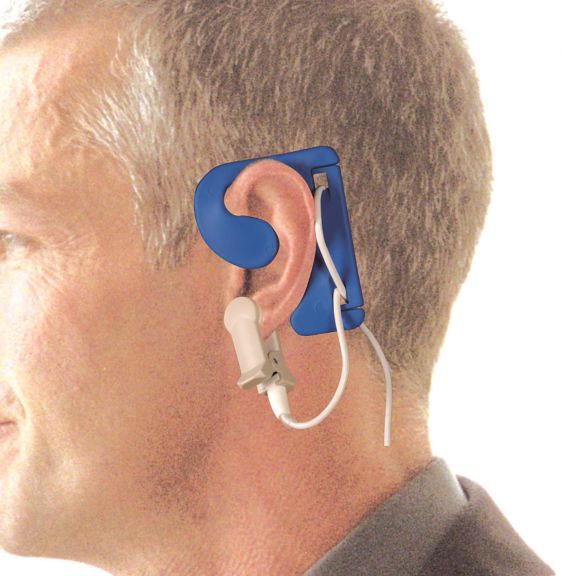
Sensores de oximetría de pulso reutilizables EarClip, ofrecen comodidad, espacio ligero y libre movimiento. Optimización del flujo de trabajo: fácil de aplicar, se queda en su lugar. Alta fiabilidad: respuesta rápida y menos artefactos de movimiento
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
Lista de referencias cruzadas de EnviteC | Soporte técnico
El uso de una sonda de SpO2 para la monitorización no invasiva de la saturación de oxígeno arterial es un tratamiento estándar en la mayoría de los entornos hospitalarios y otros entornos médicos.
Las sondas más utilizadas se colocan en la yema del dedo del paciente. Pero hay otras formas de posicionamiento de la sonda que proporcionan una buena práctica clínica y ofrecen alternativas, especialmente cuando las condiciones son malas.
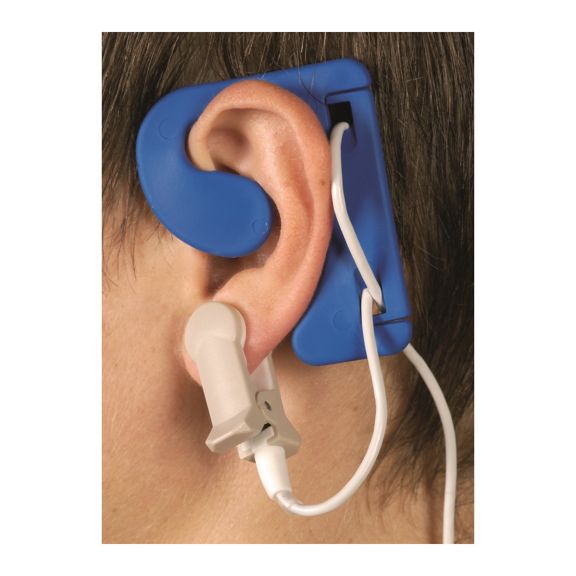
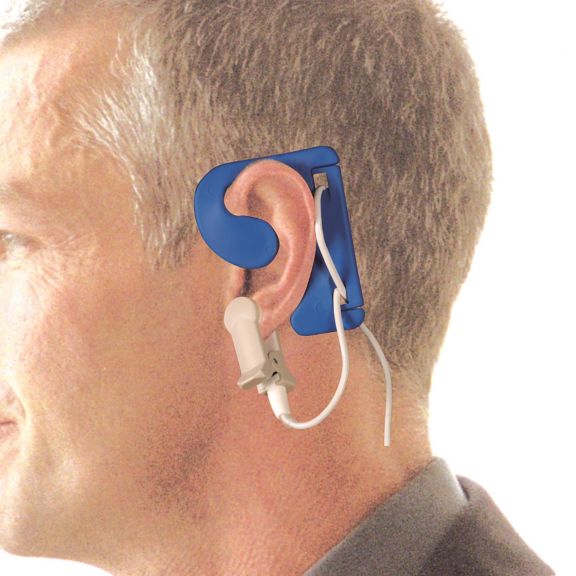
El sensor EarClip es un punto de medición probado y utilizado clínicamente. Especialmente con respecto a la precisión clínica, el EarClip no solo es equivalente al FingerClip, sino que en algunos casos incluso se ha notificado que es superior.
Para niños y adultos, el lóbulo del oído puede ser el punto de medición preferido debido a la menor interferencia de los artefactos de movimiento.
EnviteC by Honeywell
Folleto
Certificado
Otros
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión
Filters
Por favor inicie sesión para ver los números de piezas disponibles para comprar según su cuenta Iniciar sesión