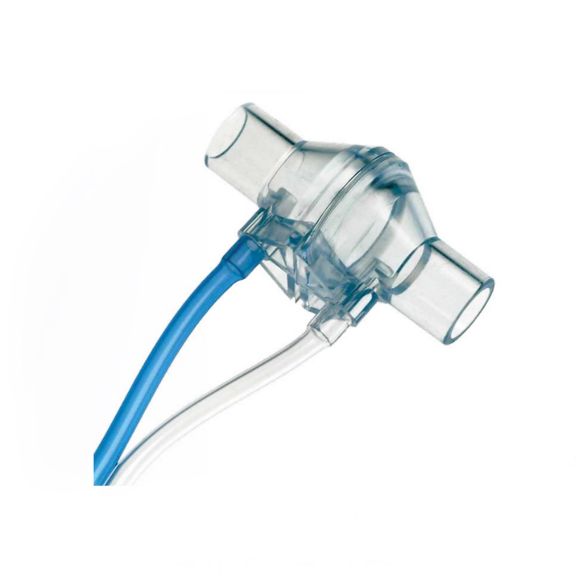
Durchflusssensoren
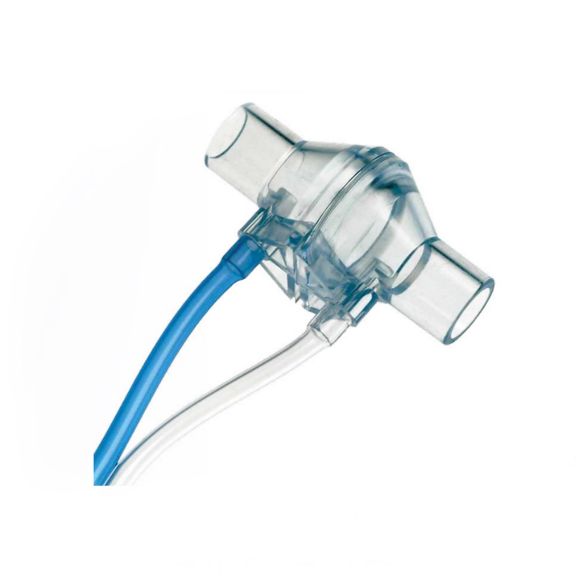
Durchflusssensoren Typ H
Typ H sind bewährte, passiv durchströmte Diffentialdruckwandler. Messung des Atemgasdurchflusses in Erwachsenenanwendungen. Hohe Produktqualität. RoHS-konform. Biokompatible Komponenten
Please sign in to access more documents
Once signed in, you may be able to access additional documents for your account.
EnviteC-Querverweisliste | Technische Unterstützung
Der passive Durchfluss-Differenzdrucksensor Typ H, der in lebenserhaltenden Systemen in der Intensiv- und Notfallmedizin eingesetzt wird, erfordert Produkte, die in jedem Detail von hoher Qualität sind. Diese Sensoren sind bekannt für ihre außergewöhnliche Präzision und Zuverlässigkeit.
EnviteC Durchflusssensoren setzen sowohl Qualitäts- als auch Leistungsstandards für präzise Messungen bei der Erkennung von Leckagen in den Atmungsorganen, bei der Erkennung von falschen Einstellungen des Beatmungsgerätes, bei der Überwachung des Beatmungsgerätes und der Beatmung sowie bei der Einstellung des Tidalvolumens während der Beatmungstherapie.
EnviteC by Honeywell
Datenblatt
Handbücher und Anleitungen
Zertifikat
Andere
Um die auf Ihr Konto abgestimmten verfügbaren Artikelnummern für den Kauf einzusehen, melden Sie sich bitte an Anmelden
Filters
Um die auf Ihr Konto abgestimmten verfügbaren Artikelnummern für den Kauf einzusehen, melden Sie sich bitte an Anmelden